The role of small molecules in stem cell biology - mini-review
 Overview
Overview
Stem cells have many potential applications in medicine - ranging from their inclusion in disease modeling and drug discovery to cell transplantation and regenerative therapies. Challenges in the field include developing methods to control stem cell differentiation, allogeneic rejection and limited cell availability. A growing range of small molecules has been identified that can be used both in vitro and in vivo as tools to expand stem cells, direct their differentiation, or reprogram somatic cells to a more naive state. These molecules can also provide useful information regarding the signaling and epigenetic mechanisms that regulate stem cell biology. This mini review highlights the applications and advantages of small molecules in key areas of stem cell biology including reprogramming, self renewal, differentiation and proliferation.
- Advantages of using small molecules in stem cell biology
- How can small molecules manipulate stem cell fate?
- Use of small molecules for reprogramming
- Effects of small molecules on self-renewal
- Small molecules can increase the survival of single cells
- Small molecules can control differentiation and proliferation
Stem cells – the basics
Stem cells are unspecialized cells that are characterized by their ability to self-renew and differentiate. They can divide into cells that bear characteristics identical to themselves (self-renewal), or they can change into specialized cells with a more limited developmental potential, (differentiation). Stem cells exist both in embryos and adults. Stem cells that are derived from distinct developmental stages may display different developmental potential:
- Totipotent stem cells have the potential to generate an entire functional organism, including not only the embryo but also the extra-embryonic tissues. Examples of totipotent cells are the fertilized eggs and early embryonic cells of mammals.
- Pluripotent stem cells (PSCs) can give rise to all the cell types of the entire embryo but not the extraembryonic tissues, such as placenta. Examples of pluripotent stem cells include:
embryonic stem cells (ESCs): derived from the inner cell mass of preimplantation embryos 1, and epiblast stem cells (EpiSCs) derived from the epiblast layer of the implanted embryos 2 - Induced pluripotent stem cells (iPSCs): generated from somatic cells by reprogramming 3
- Multipotent stem cells have the capacity to develop into different cell types within the same cell lineage and are, therefore, also referred to as lineage-specific stem cells or progenitors. An example would be hematopoietic stem cells in bone marrow, which can give rise to all types of blood cell and replenish peripheral blood.
The advantages of using small molecules in stem cell biology
Over recent years, small molecules have emerged as essential tools for understanding and regulating stem cells, and manipulating stem cell fate. They can have wide-ranging effects - from reprogramming, expansion or directed differentiation of stem cells, to therapeutic effects in in vivo disease models, and survival, ablation, or migration of cancer cells.
Small molecules have advantages over genetic and other methods:
- they are able to reversibly alter specific functions of a single protein (or multiple proteins) with good temporal control. This is a useful feature, as differentiation into a given lineage is dependent upon a specific sequence of cellular events.
- they can be used in primary cell assays and easily adapted into in vivo models
- small molecules can be cell permeable and affect signaling pathways and processes within the cell
- they are more stable and cost-effective than growth factors
- small molecules can be of high purity and provide robust reproducible results, with little batch to batch variation in activity
- the actions of small molecules are concentration dependent – allowing flexibility in the effects achieved
- Small molecules can be used as chemical probes to further our understanding of the mechanisms that control developmental potential and cell fate.
How can small molecules manipulate stem cell fate?
The adjacent figure shows the processes that can be affected by small molecules and that can modify or manipulate stem cell fate. Small molecules can modify stem cell self-renewal, and induction of lineage-specific differentiation. They can also have an impact on iPSC reprogramming - they can replace certain transcription factors, enhance the efficiency of reprogramming or accelerate the reprogramming process. The conversion of primed pluripotent stem cells into naive stem cells can also be facilitated by small molecules.
Transdifferentiation can occur when one somatic cell type converts into another, bypassing pluripotency. It can be mediated either by lineage-specific factors (transdifferentiation I in the figure below) or the restricted reprogramming and subsequent lineage-specific differentiation (transdifferentiation II in the figure below). This latter process can also potentially be affected by small molecules known to be involved in reprogramming and differentiation (see 4 for review)
Use of small molecules for reprogramming
iPS cells have traditionally been generated through exogenous expression of pluripotency genes via viral or episomal vectors but these methods are inefficient, taking weeks to produce small numbers of cells. Additionally, for iPS cell-based therapies to be considered for use in medicine, the use of retroviruses and transcription factors associated with tumorigenesis must also be eliminated. Small molecules can greatly enhance the efficiency of generating iPS cell lines and also reduce or eliminate the need for genetic factors:
For example, PD0325901, Thiazovivin, and SB431542 used in combination, can significantly increase the efficiency of reprogramming human fibroblasts to iPS cells 5.
HDAC inhibitors Valproic acid and Trichostatin A both enhance the yield of iPS cells using the four-factor dedifferentiation method. Using the DNA methyl transferase inhibitor, 5-azacy tidine, can result in a yield 100-fold higher than the four-factor method alone 6.
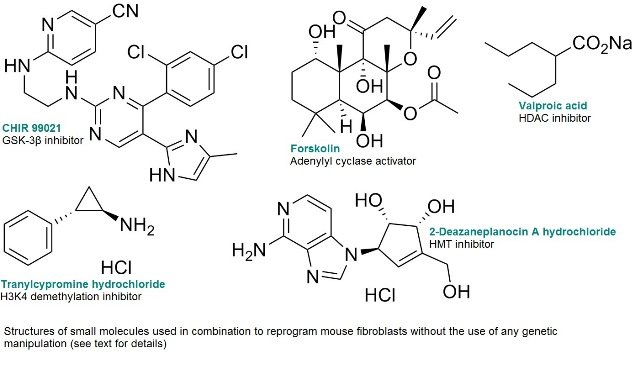
Small molecules have been shown to be useful in generating high quality iPSCs without genetic defects, preserving the genomic integrity by facilitating the reprogramming process 8.
Reducing the reliance on genetic methods, it is possible to reprogram mouse fibroblasts without the use of any genetic manipulation by using a combination of small molecules including CHIR99021, Forskolin, Tranylcypromine, Valproic Acid, and 3-Deazaneplanocin A9.
Table 1: Small molecules that promote somatic cell reprogramming
M=mouse; H=human
| Cat No | Name | Compound overview | Effects on reprogramming | Concentration | Host animal | References |
| TGFβ receptor inhibitors | ||||||
| HB3301 | RepSox (E-616452) | TGF-β inhibitor | Able to replace Sox2 | 1 μM | M,H | 10, 11 |
| HB3218 | A 83-01 | TGF-β inhibitor | Enhances reprogramming | 0.5 μM | M,H | 12, 13 |
| HB3555 | SB 431542 | TGF-β inhibitor | Enhances reprogramming | 2 μM | M,H | 14 |
| GSK-3β inhibitors | ||||||
| HB1261 | CHIR 99021 | GSK-3β inhibitor and Wnt signaling activator | Able to replace Sox2 | 3-10 μM | M,H | 14, 15 |
| HB1266 | Kenpaullone | GSK-3/CDKs inhibitor | Able to replace Klf4 | 5 μM | M | 16 |
| MEK inhibitors | ||||||
| HB2240 | PD 0325901 | MEK inhibitor | Enhances reprogramming | 0.5-1 μM | M,H | 17 |
| cAMP agonists | ||||||
| HB1348 | Forskolin | Adenylyl cyclase agonist. Increases cAMP levels. | Able to replace Oct4 (with 2-Me-5HT & D4476) | M | 9 | |
| HB3460 | Prostaglandin E2 | Prostaglandin E2 receptor 4 agonist. Increases cAMP levels. | Enhances reprogramming | 5 μM | M | 9 |
| Sonic hedgehog signaling activators | ||||||
| HB3179 | JK 184 (Shh antagonist) | Sonic hedgehog signaling inhibitor | Able to replace Sox2, Klf4, c-Myc | 500 ng/ml | M | 18 |
| Histone deacetylase (HDAC) inhibitors | ||||||
| HB1399 | Sodium butyrate (NaB) | HDAC inhibitor | Enhances reprogramming | 0.5-1 mM | M,H | 19 |
| HB0867 | Valproic acid sodium salt (VPA) | HDAC inhibitor | Enhances reprogramming. Also able to replace c-Myc/Klf4 in human fibroblasts | 0.5-2 mM | M,H | 20, 7, 15 |
| HB1396 | SAHA | HDAC inhibitor | Enhances reprogramming | 5 μM | M | 20 |
| HB1402 | Trichostatin A (TSA) | HDAC inhibitor | Enhances reprogramming | 20 nM | M | 20 |
| HB1412 | Tranylcypromine hydrochloride (Parnate) | H3K4 demethylation inhibitor | Enhances reprogramming | 5-10 μM | M | 14 |
| HB1356 | 5-aza-2'-deoxycytidine (Decitabine) | DNMT inhibitor | Enhances reprogramming | 0.5 mM | M | 21, 22 |
| HB1377 | RG 108 | DNMT inhibitor | Able to replace Sox2 (with BIX 01294) or Oct4 | 0.04-500 μM | M | 23, 24, 14 |
| Histone methyltransferase (HMT) inhibitor | ||||||
| HB1413 | BIX 01294 | G9a HMTase inhibitor | Enhances reprogramming. Also able to replace Oct4 | 0.5-2 μM | M | 17 |
| Src family tyrosine kinase inhibitors | ||||||
| HB1334 | PP 1 | Src family tyrosine kinase inhibitor | Able to replace Sox2 | 10 μM | M | 25 |
| Miscellaneous | ||||||
| HB0758 | AMI-5 (Eosin Y) | Protein arginine methyltransferase inhibitor | Able to replace Sox2, Klf4 (with A-83-01) | 5 μM | M | 13 |
| HB2167 | D 4476 | CK1 inhibitor | Able to replace Oct4 (with Forskolin & 2-Me-5HT) | 5 μM | M | 9 |
| HB2779 | Rapamycin | mTOR inhibitor | Enhances reprogramming | 0.3 nM | M | 26 |
| HB0542 | Quercetin | Hypoxia inducible factor (HIF) pathway activator | Enhances reprogramming | 1 μM | H | 12 |
| HB1209 | (±)-Bay K 8644 | L-type Ca2+ channel agonist | Able to replace Sox2 | 2 μM | M | 23 |
Effects of small molecules on self-renewal
Maintaining stem cells in defined culture systems is important as it helps to reduce experimental variability. To promote self renewal, it is common practice to add basic FGF into the medium, when culturing human ES cells and culture the cells on a ‘feeder layer’ of mouse embryonic fibroblasts (MEFs) or in conditioned media. However, the use of serum products and feeder layers has some disadvantages:
- Feeder layers may restrict the use of human ES cells in therapeutic settings due to concern of xenogenic contamination; increasing the variability of results, and limiting use on a large scale.
- Batch to batch variability can be a problem when using serum products
- The use of serum products and feeder layers may also bias stem cell fate toward specific lineage types, through the activation of certain signaling pathways 27
- Small molecules can reduce or eliminate the need for serum products and feeder layers. For example:
Pluripotin is a small molecule that promotes the long-term maintenance of mouse ES cells without the need for feeder layers, LIF, BMPs or Wnt proteins 28. ID 8 is another small molecule that promotes mouse ES cell proliferation in serum-free conditions29.
Small molecules such as CHIR99021 (a GSK-3β inhibitor that supresses the Wnt pathway), PD0325901 (which inhibits the MEK pathway), and SB 203580 can stimulate self-renewal of embryonic stem (ES) cells and induced pluripotent stem (iPS) cells30.
Table 2: Small molecules that affect pluripotent stem cell self-renewal and maintenance
| Cat No | Name | Overview | Target Cell |
| HB1261 | CHIR 99021 | Inhibits GSK-3. Promotes self-renewal. | mESC |
| HB1259 | BIO | Inhibits GSK-3. Activates Wnt signaling and maintains ESC self-renewal. | hESC and mESC |
| HB3240 | Thiazovivin | Inhibits ROCK. Enhances ESC survival. | hESC |
| HB3133 | SU 5402 | Inhibits FGFRR. Maintains self-renewal. | mESC |
| HB2223 | Pluripotin (SC-1) | Inhibits RasGAP and ERK1. Promotes self-renewal. | mESC |
| HB1302 | SB 203580 | Inhibits P38-MAPK. Promotes mESC survival. | mESC |
| HB3282 | IQ 1 | Binds to PP2A. Decreases p300 phosphorylation and maintains mES cells in an undifferentiated state. | mESC |
 Small molecules can increase the survival of single cells
Small molecules can increase the survival of single cells
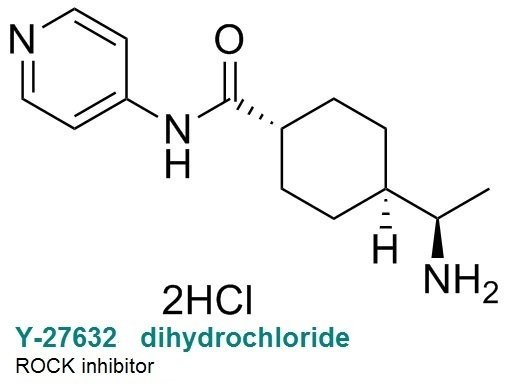
The low viability of single cells in cultured human pluripotent stem cells makes genome-editing and cloning techniques difficult. ROCK inhibitors Y-27632 32 and Thiazovivin have been shown to increase survival of single human embryonic stem cells through inhibition of RHO/ROCK signaling31.
Small molecules can control differentiation and proliferation
Small molecules provide an effective way of controlling cellular differentiation, reducing the need for more expensive growth factors. By using small molecules to selectively activate and inhibit specific developmental signalling pathways, it is possible to induce differentiation of pluripotent stem cells to specialized cell types. These developmental pathways include:
The retinoic acid pathway
The synthetic retinoid EC23 is an example of a small molecule, acting through this pathway, that is a potent inducer of stem cell differentiation33.
 Transforming Growth Factor-β Superfamily
Transforming Growth Factor-β Superfamily
SB431542 is an inhibitor that targets the TGF-β superfamily and is an important tool in stem cell biology – it acts by inhibiting activin receptor-like kinases 4, 5 and 7 (ALK4, TGF-βR1 and ALK7 respectively), and has effects on proliferation, differentiation and promotion of sheet formation of endothelial cells derived from ES cells34. It also promotes differentiation of glioblastoma CS cells11. More recently it has been shown that inhibition of Activin/Nodal/TGF-β and BMP signaling pathways by SB431542 together with Dorsomorphin induces neuronal differentiation of human adipose derived stem cells35.
Canonical Wnt Pathway
This signaling pathway has a significant and well documented role in proliferation, self-renewal and differentiation of stem cells36, 37. Abberation of this pathway can result in tumourigenesis, due to increased activation leading to increased cellular proliferation.
In terms of small molecules, GSK-3β inhibitor CHIR99021 is commonly used to efficiently direct human pluripotent stem cells (hPSCs) to functional cardiomyocytes in a completely defined, growth factor- and serum-free system by modulating canonical Wnt signalling38. IWP-2, another WNT pathway inhibitor induces cardiomyocyte differentiation in pluripotent stem cell-generated mesoderm38.
Kunisada and colleagues developed a protocol for generating insulin-producing cells from hiPS cells and showed that treatment with Activin A and CHIR99021 enhanced efficient endodermal differentiation. They also demonstrated that small molecules Forskolin, Dexamethasone, and SB431542 (a TGF-β inhibitor), were able to induce the differentiation of insulin-producing cells from pancreatic progenitor cells39.
Notch signaling pathway
Notch pathway inhibitor DAPT has recently been shown to promote differentiation of neural stem/progenitor cells into neurons40 and to promote cardiac differentiation of murine pluripotent stem cells41.
Other signaling pathways: Hedgehog and FGFs
The Hedgehog signaling pathway: Hedgehog (Hh) signaling is essential for self-renewal and cell fate determination. Dysfunctional Hh signaling is associated with the development and progression of various types of cancer and is implicated in multiple aspects of tumourigenesis, including the maintenance of cancer stem cells42. Examples of small molecules that act on Hh signaling pathways include Cyclopamine and GANT61.
Fibroblast growth factors (FGFs) play a key role in the proliferation and differentiation of a variety of cells, and can activate the MAPK/ERK pathway through MEK (MAPK/ERK kinase) activation. PD0325901 a small molecule MEK (MAP3 kinase) inhibitor, promotes the efficiency of mouse and human iPSC (miPSC, hiPSC) reprogramming and late somatic cell reprogramming (after Oct4 activation). PD0325901 also inhibits the growth of non-iPSC colonies and supports the growth of reprogrammed iPSCs43. Small molecule inhibitors or the FGF receptor itself include PD 173074.
Table 3: Small molecules that regulate differentiation
| Cat No | Name | Overview | Target Cell |
| HB2266 | LY 294002 hydrochloride | Inhibits PI3K. Promotes differentiation to mesoderm. | hESC and mESC |
| HB2800 | Dorsomorphin dihydrochloride (Compound C) | Inhibits BMP. Promotes neural differentiation. | hESC and hiPSC |
| HB1237 | Verapamil hydrochloride | Blocks L-type Ca2+ channels. Promotes cardiomyocyte differentiation. | mESC |
| HB3011 | Stauprimide | Inhibits NME2 nuclear localization and downregulates c-Myc. Enhances endoderm, ectoderm and mesoderm differentiation. | mESC and hESC |
| HB0002 | (-)-Indolactam V | Activates PKC signaling. Promotes pancreatic differentiation. | hESC and mESC |
Targeting multiple pathways – cocktails of small molecules
In many instances, combining small molecules targeting multiple pathways essential for development has been shown to be an effective approach for inducing differentiation. For example, a combination of SB431542, LDN-193189, CHIR99021, and SU5402 (inhibiting FGFR and VEGFR), and DAPT (inhibiting γ-secretase) induced differentiation of hPSCs into nociceptor neurons in a much accelerated manner with >75% efficiency within 10 days44.
Summary
To summarise, the development and use of small molecules is an exciting branch of stem cell biology – offering the researcher opportunities to improve reprogramming efficiency, self-renewal, control differentiation and proliferation as well as providing crucial insights into the signaling and epigenetic mechanisms that regulate stem cell biology.
References
1. Thomson JA et al (1998) Embryonic stem cell lines derived from human blastocysts. Science 282(5391) 1145-7. Pubmed ID:9804556
2. Brons IG et al (2007) Derivation of pluripotent epiblast stem cells from mammalian embryos. Nature 448(7150) 191-5. Pubmed ID:17597762
3. Takahashi K et al (2007) Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell 131(5) 861-72. Pubmed ID:18035408
4. Zhang Y et al (2012) Small molecules, big roles -- the chemical manipulation of stem cell fate and somatic cell reprogramming. J Cell Sci 125(Pt 23) 5609-20. Pubmed ID:23420199
5. Lin T et al (2009) A chemical platform for improved induction of human iPSCs. Nat Methods 6(11) 805-8. Pubmed ID:19838168
6. Tsuji-Takayama K et al (2004) Demethylating agent, 5-azacytidine, reverses differentiation of embryonic stem cells. Biochem Biophys Res Commun 323(1) 86-90. Pubmed ID:15351705
7. Huangfu D et al (2008) Induction of pluripotent stem cells from primary human fibroblasts with only Oct4 and Sox2. Nat Biotechnol 26(11) 1269-75. Pubmed ID:18849973
8. Park HS et al (2015) Generation of induced pluripotent stem cells without genetic defects by small molecules. Biomaterials 39 47-58. Pubmed ID:25477171
9. Hou P et al (2013) Pluripotent stem cells induced from mouse somatic cells by small-molecule compounds. Science 341(6146) 651-4. Pubmed ID:23868920
10. Attisano L et al (2002) Signal transduction by the TGF-beta superfamily. Science 296(5573) 1646-7. Pubmed ID:12040180
11. Ichida JK et al (2009) A small-molecule inhibitor of tgf-Beta signaling replaces sox2 in reprogramming by inducing nanog. Cell Stem Cell 5(5) 491-503. Pubmed ID:19818703
12. Zhu S et al (2010) Reprogramming of human primary somatic cells by OCT4 and chemical compounds. Cell Stem Cell 7(6) 651-5. Pubmed ID:21112560
13. Yuan X et al (2011) Brief report: combined chemical treatment enables Oct4-induced reprogramming from mouse embryonic fibroblasts. Stem Cells 29(3) 549-53. Pubmed ID:21425417
14. Li W et al (2009) Generation of human-induced pluripotent stem cells in the absence of exogenous Sox2. Stem Cells 27(12) 2992-3000. Pubmed ID:19839055
15. Li Y et al (2011) Generation of iPSCs from mouse fibroblasts with a single gene, Oct4, and small molecules. Cell Res 21(1) 196-204. Pubmed ID:20956998
16. Lyssiotis CA et al (2009) Reprogramming of murine fibroblasts to induced pluripotent stem cells with chemical complementation of Klf4. Proc Natl Acad Sci U S A 106(22) 8912-7. Pubmed ID:19447925
17. Shi Y et al (2008) A combined chemical and genetic approach for the generation of induced pluripotent stem cells. Cell Stem Cell 2(6) 525-8. Pubmed ID:18522845
18. Moon JH et al (2011) Reprogramming fibroblasts into induced pluripotent stem cells with Bmi1. Cell Res 21(9) 1305-15. Pubmed ID:21709693
19. Mali P et al (2010) Butyrate greatly enhances derivation of human induced pluripotent stem cells by promoting epigenetic remodeling and the expression of pluripotency-associated genes. Stem Cells 28(4) 713-20. Pubmed ID:20201064
20. Huangfu D et al (2008) Induction of pluripotent stem cells by defined factors is greatly improved by small-molecule compounds. Nat Biotechnol 26(7) 795-7. Pubmed ID:18568017
21. Mikkelsen TS et al (2008) Dissecting direct reprogramming through integrative genomic analysis. Nature 454(7200) 49-55. Pubmed ID:18509334
22. Papp B et al (2013) Epigenetics of reprogramming to induced pluripotency. Cell 152(6) 1324-43. Pubmed ID:23498940
23. Shi Y et al (2008) Induction of pluripotent stem cells from mouse embryonic fibroblasts by Oct4 and Klf4 with small-molecule compounds. Cell Stem Cell 3(5) 568-74. Pubmed ID:18983970
24. Li W et al (2010) Small molecules that modulate embryonic stem cell fate and somatic cell reprogramming. Trends Pharmacol Sci 31(1) 36-45. Pubmed ID:19896224
25. Staerk J et al (2011) Pan-Src family kinase inhibitors replace Sox2 during the direct reprogramming of somatic cells. Angew Chem Int Ed Engl 50(25) 5734-6. Pubmed ID:21547985
26. Chen T et al (2011) Rapamycin and other longevity-promoting compounds enhance the generation of mouse induced pluripotent stem cells. Aging Cell 10(5) 908-11. Pubmed ID:21615676
27. Xu Y et al (2008) A chemical approach to stem-cell biology and regenerative medicine. Nature 453(7193) 338-44. Pubmed ID:18480815
28. Chen S et al (2006) Self-renewal of embryonic stem cells by a small molecule. Proc Natl Acad Sci U S A 103(46) 17266-71. Pubmed ID:17088537
29. Miyabayashi T et al (2008) Indole derivatives sustain embryonic stem cell self-renewal in long-term culture. Biosci Biotechnol Biochem 72(5) 1242-8. Pubmed ID:18460821
30. Li W et al (2009) Generation of rat and human induced pluripotent stem cells by combining genetic reprogramming and chemical inhibitors. Cell Stem Cell 4(1) 16-9. Pubmed ID:19097958
31. Xu Y et al (2010) Revealing a core signaling regulatory mechanism for pluripotent stem cell survival and self-renewal by small molecules. Proc Natl Acad Sci U S A 107(18) 8129-34. Pubmed ID:20406903
32. Watanabe et al (2007) A ROCK inhibitor permits survival of dissociated human embryonic stem cells.Nat Biotechnol. 2007 25:681-6 Pubmed ID:17529971
33. Christie VB et al (2008) Synthesis and evaluation of synthetic retinoid derivatives as inducers of stem cell differentiation. Org Biomol Chem 6(19) 3497-507. Pubmed ID:19082150
34. Watabe T et al (2003) TGF-beta receptor kinase inhibitor enhances growth and integrity of embryonic stem cell-derived endothelial cells. J Cell Biol 163(6) 1303-11. Pubmed ID:14676305
35. Madhu V et al (2016) Dual Inhibition of Activin/Nodal/TGF-beta and BMP Signaling Pathways by SB431542 and Dorsomorphin Induces Neuronal Differentiation of Human Adipose Derived Stem Cells. Stem Cells Int 2016 1035374 Pubmed ID:26798350
36. Mohammed MK et al (2016) Wnt/beta-catenin signaling plays an ever-expanding role in stem cell self-renewal, tumorigenesis and cancer chemoresistance. Genes Dis 3(1) 11-40. Pubmed ID:27077077
37. Yang K et al (2016) The evolving roles of canonical WNT signaling in stem cells and tumorigenesis: implications in targeted cancer therapies. Lab Invest 96(2) 116-36. Pubmed ID:26618721
38. Lian X et al (2013) Directed cardiomyocyte differentiation from human pluripotent stem cells by modulating Wnt/beta-catenin signaling under fully defined conditions. Nat Protoc 8(1) 162-75. Pubmed ID:23257984
39. Kunisada Y et al (2012) Small molecules induce efficient differentiation into insulin-producing cells from human induced pluripotent stem cells. Stem Cell Res 8(2) 274-84. Pubmed ID:22056147
40. Wang J et al (2016) Lingo-1 shRNA and Notch signaling inhibitor DAPT promote differentiation of neural stem/progenitor cells into neurons. Brain Res 1634 34-44. Pubmed ID:26607252
41. Liu Y et al (2014) Timely inhibition of Notch signaling by DAPT promotes cardiac differentiation of murine pluripotent stem cells. PLoS One 9(10) e109588. Pubmed ID:25313563
42. Cochrane CR et al (2015) Hedgehog Signaling in the Maintenance of Cancer Stem Cells. Cancers (Basel) 7(3) 1554-85. Pubmed ID:26270676
43. Silva J et al (2008) Promotion of reprogramming to ground state pluripotency by signal inhibition. PLoS Biol 6(10) e253. Pubmed ID:18942890
44. Chambers SM et al (2012) Combined small-molecule inhibition accelerates developmental timing and converts human pluripotent stem cells into nociceptors. Nat Biotechnol 30(7) 715-20. Pubmed ID:22750882
45. Davies SG et al (2015) Stemistry: the control of stem cells in situ using chemistry. J Med Chem 58(7)2863-94. Pubmed ID:25590360





 Overview
Overview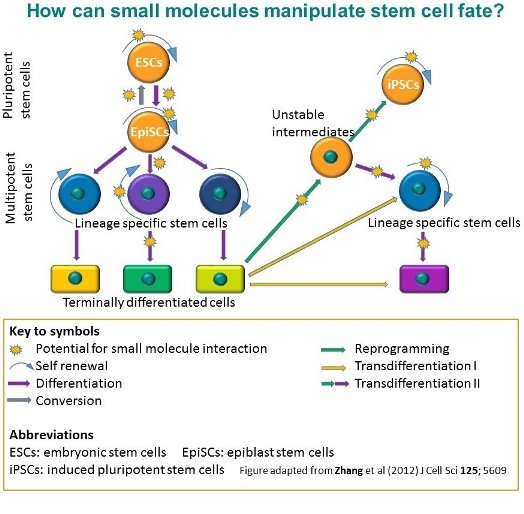
 Small molecules can increase the survival of single cells
Small molecules can increase the survival of single cells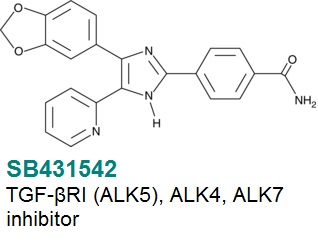 Transforming Growth Factor-β Superfamily
Transforming Growth Factor-β Superfamily