Understanding purity and quality - a guide for life scientists
 Overview
Overview
There are many suppliers of biochemicals, and not all of them have their own manufacturing capabilities – so how do you know that the little vial of powder that arrives in your lab, is what you think it is, and that it is of high enough quality for your requirements?
This mini-review is written by our own expert team of chemists and biologists, who have a wealth of synthetic experience in a wide variety of commercial and pharmaceutical settings. Tailored to life scientists, the review provides a few key points to help you verify the quality of your product, and guides you through the chemical jargon and techniques that are used to assess biochemical purity and quality.
Although not exhaustive, we hope that this mini-review will go some way to 'de-mystifying' the techniques and methods used to determine chemical purity and quality - and provide the life scientist with a means of more critically assessing the quality of their products. Topics covered include:
- Carrying out your own checks
- The value of a Certificate of Analysis
- Understanding the chemical data
- Laboratory techniques commonly used to assess chemical structure, purity and quality of biochemicals
- Certificate of Analysis - the chemical terms explained
- Biological testing
Carrying out your own ‘pre-flight’ checks
For many life scientists, when they order a biochemical from a life science supplier, it is taken on trust that it is the right product, and of the required quality. However, many suppliers do not manufacture their products in-house. And - whilst Hello Bio has its own manufacturing facility, and stringent and rigorous quality control standards, as with all industries, other suppliers do differ widely in their standards. Therefore there are a few basic checks that you should carry out yourself when you receive a biochemical product, to give you confidence that the product in your vial is what you think it is.
Check 1: Read the product datasheet

Check 2: Visually inspect your product
It may sound obvious, but a quick look at what is in your vial is an important first step. If the datasheet says that it should be a white powder – then check that it is! But you need to be aware that some products are supplied in very small quantities or as lyophilized solids, and may be extremely difficult to see – they may appear as a clear, thin film on the bottom and walls of the vial. Again, this should be indicated on your datasheet so check that it is.
Check 3: Product characteristics
Be alert to any unusual characteristics of your product – check that the solubility matches that stated on the datasheet. If the datasheet states that it should be soluble to 100 mM in water – then it should be. However, be aware, that you need to work harder to get some products in solution than others – so follow the datasheet instructions or give your technical help team a call.
Check 4: Purity
A figure for purity should be stated on your datasheet – and for robust, reproducible results, you should check that this is broadly similar to previous batches of the product that you have used in the past. In general, for biochemicals you would ideally be looking for >98% purity. This means that you would have 2% or less of impurities in your product. However, due to the chemical nature of some products (mixtures, plant extracts etc) – a purity of >98% may not be possible or appropriate, or may even be expressed in different ways (eg. 98% of a mixture of A and B). For example, due to the synthetic processes used peptides products are usually synthesised at a purity of > 95%.
The value of a Certificate of Analysis
The Certificate of Analysis (CoA) is a more detailed document, which includes the methods used and data obtained during the Quality Control (QC) procedures. For most life scientists, the datasheet is usually sufficient, but the CoA is of value for those researchers requiring more reassurance and data about the quality of their product. Helpful tips on reading and understanding this document are included later in this article.
Understanding the chemical data
So – you have checked that your product matches the specification document as far as you are able, but how do you make sense of all of the chemical data that you are supplied with? What are the techniques that are used, and what does all of the information mean?
To determine chemical structures, chemists use a variety of spectroscopic techniques, such as Infrared spectroscopy (IR), Mass Spectrometry (MS), UV-VIS spectroscopy (UV), and Nuclear Magnetic Resonance spectroscopy (NMR). Other techniques include HPLC, chiral HPLC, microanalysis and optical rotation. Chemists generally use a combination of methods and techniques to build up a purity and quality profile of the chemical, and to confirm that the chemical is what it is meant to be. At Hello Bio, these checks are carried out every time a new batch is synthesized.
Laboratory techniques commonly used to assess chemical structure, purity and quality of biochemicals
The table below summarises the techniques used by chemists to identify and assess the purity and quality of biochemicals.
| Name of Technique | How does it work? | What is it used for? | What does it tell us? |
| High Performance Liquid Chromatography (HPLC) |
|
|
We use this method to determine the purity of our products. The ratio of the desired product to that of the combined impurities is expressed as a % purity. We will typically state that a product is say >98% (by HPLC). |
| Nuclear Magnetic Resonance (1H-NMR) |
|
|
We will compare the data obtained from analysis of our product to that presented in the literature, predicted information or analysis of a known standard. If the two match then we will describe our result as 'Meets specification'. |
| Mass Spectrometry | The mass of a molecule and molecular fragments is measured by ionising it and passing a beam of ionised molecules through an electric field into a detector. | Can be used to determine the molecular weight of the compound and to obtain structural information. | We will attempt to identify the ionised molecule. The results will look something like [M+H]+ = 123.4. This means that we have identified a peak that corresponds to the molecular weight of the desired compound plus a proton. |
| Microanalysis | A sample is thoroughly oxidised by combustion and the product gases are separated and detected. |
|
We will determine the ratios of C,H,N and possibly other relevant elements and compare the values to the theoretical values. If the results closely match (within 0.4%) then we will record the result as ‘Meets specification’ |
| Optical rotation |
|
Where a single enantiomer is the desired product, optical rotation will confirm the ratio of the desired isomer to the undesired one. | We will determine the optical rotation and compare it to that of a previous sample or known optically pure standard. This will enable us to calculate an optical purity. |
| Thin layer chromatography (TLC) |
|
Where a pure, single component product is required, TLC analysis will not result in separation and will show only one component. | We use this method to check that only one component is observed and to check that the product elutes at the correct position. |
| Physical appearance | Different products will sometimes have different appearances. But broadly speaking there should be reproducibility of the appearance between batches. | A description of the appearance will contribute to confidence in the identity of the product. | We use this to support a product identity by comparison against a previous batch or a known standard. It will usually be expressed as a physical state and a colour eg. ‘Meets Specification White Solid’ |
| Solubility | Different products will sometimes have different solubility profiles. But, there should be reproducibility between batches. | Determining the solubility will contribute to confidence in the identity of the product. | As well as providing useful information to researchers, we also use solubility information to support a product identity by comparison against a previous batch or a known standard. It will usually be expressed as the concentration to which the product will dissolve in a particular solvent. |
Certificate of Analysis - the chemical terms explained
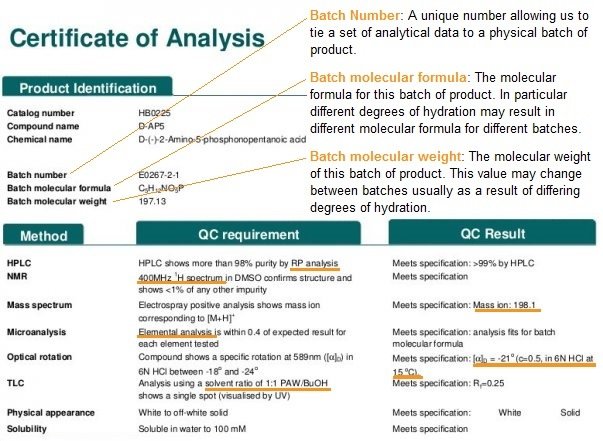
The diagram below shows a real example of a CoA for D-AP5 from Hello Bio, with a key and annotations to explain the chemistry jargon:
Key to chemical terms
|
RP analysis 400MHz 1H spectrum Elemental analysis Solvent ratio of 1:1 PAW/BuOH |
Mass ion = 198.1
[a]D=-21 (c=0.5, in 6N HCl at 15oC) |
Biological testing
An additional level of assurance for researchers is the availability of batch specific biological data – from electrophysiological or in vitro assays for example. The product should behave as expected when compared to data from the scientific literature. Electrophysiological assays are commonly used for ion channel modulators and ionotropic receptor ligands, for example, as they are quick and relatively simple, and have precedent in the literature.
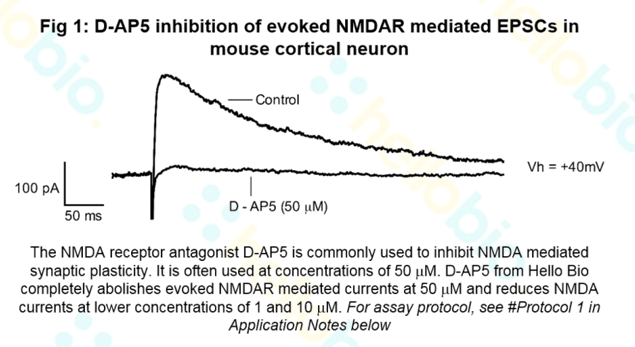
Let’s take the NMDA receptor antagonist D-AP5 as an example. This biochemical is commonly used to inhibit NMDA mediated synaptic plasticity and is often used at concentrations of 50 μM. As part of the Hello Bio Quality Control process, the effects of D-AP5 on evoked NMDA receptor EPSCs in mouse cortical neurons are tested at a range of concentrations.
In the example below, D-AP5 from Hello Bio completely abolishes evoked NMDAR mediated currents at 50 μM and reduces NMDA currents at lower concentrations of 1 and 10 μM, in keeping with the scientific literature. The details of the protocols used, and results are provided on the datasheet and the Certificate of Analysis
Building a profile of your product – and your confidence in it
So the quality of a product is determined from a whole host of chemical and biological techniques including mass spectrometry, nuclear magnetic resonance spectroscopy (NMR), HPLC, chiral HPLC, microanalysis, optical rotation and electrophysiological techniques. The results of these techniques each serve to build a 'quality picture' of your product – if all the elements are as expected, then you should have a high quality, bioactive product, giving robust, reproducible results. This is of course, assuming that the scientific protocols are also robust, and that personnel are carrying them out correctly – but that is beyond the scope of this article!
For any questions on the purity and quality of your product our Technical Help team will be happy to help.





 Overview
Overview