Anti-NeuN antibody ValidAb™
Certificate of Analysis
Latest Certificate of Analysis
Download the latest CoA for this product:
Download CoADownload Batch-Specific CoA
To download the CoA for a batch, enter the batch ID below:
Product overview
| Name | Anti-NeuN antibody ValidAb™ |
| Alternative names | Fox-3 |
| Host | Mouse |
| Clonality | Monoclonal |
| Target | NeuN |
| Customer comments | The NeuN antibody shows good specificity and signal/noise (S/N). At equivalent dilution, the signal is brighter with this antibody than with our usual antibodies - the Poncer lab, Institute Du Fer À Moulin - Inserm. |
| Description | Antibody to NeuN - marker for mature neurones expressed in the nucleus. Part of the ValidAb™ range of highly validated, data-rich antibodies. |
Validation data
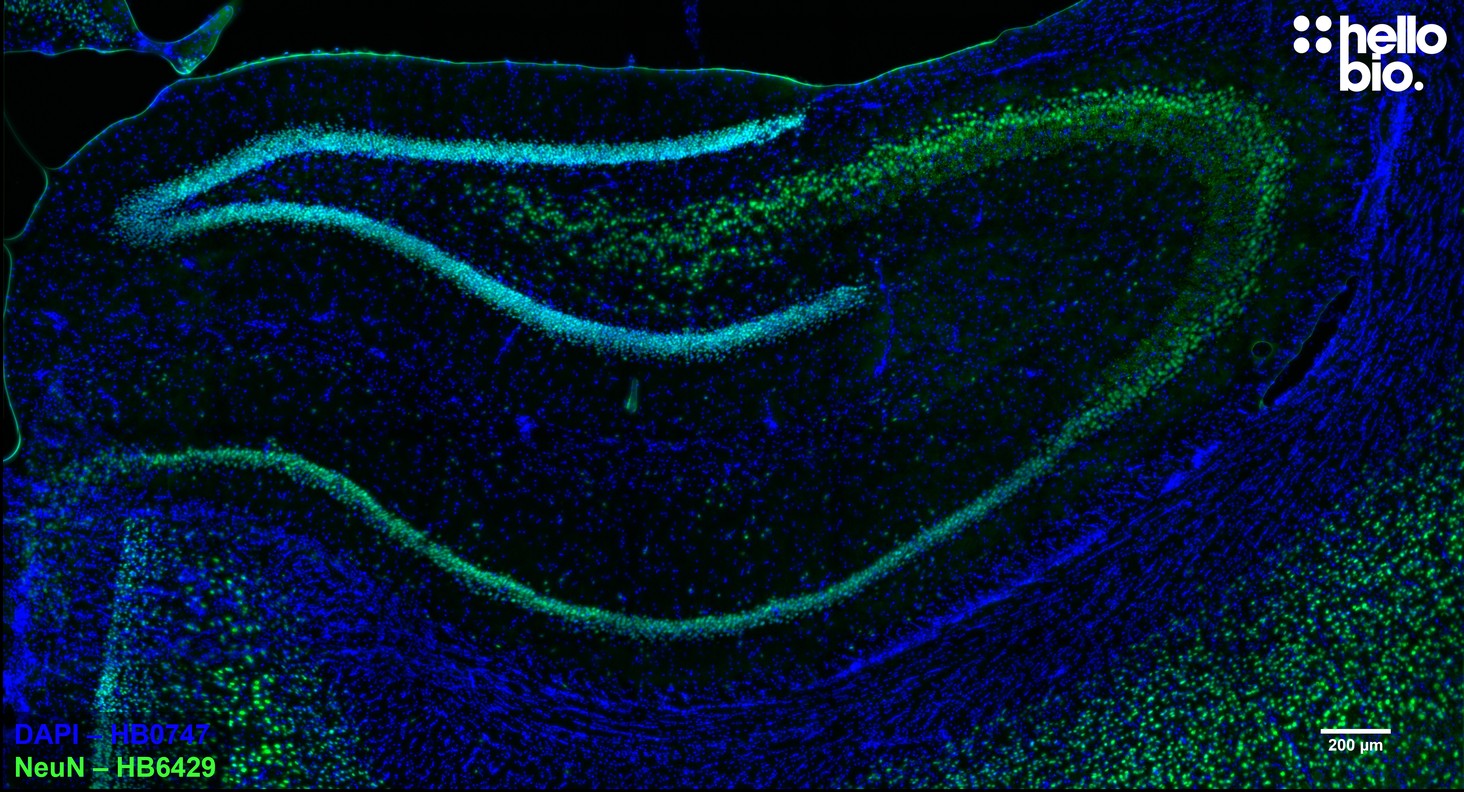
Figure 1. HB6429 staining of NeuN expressing neurons in rat hippocampus.
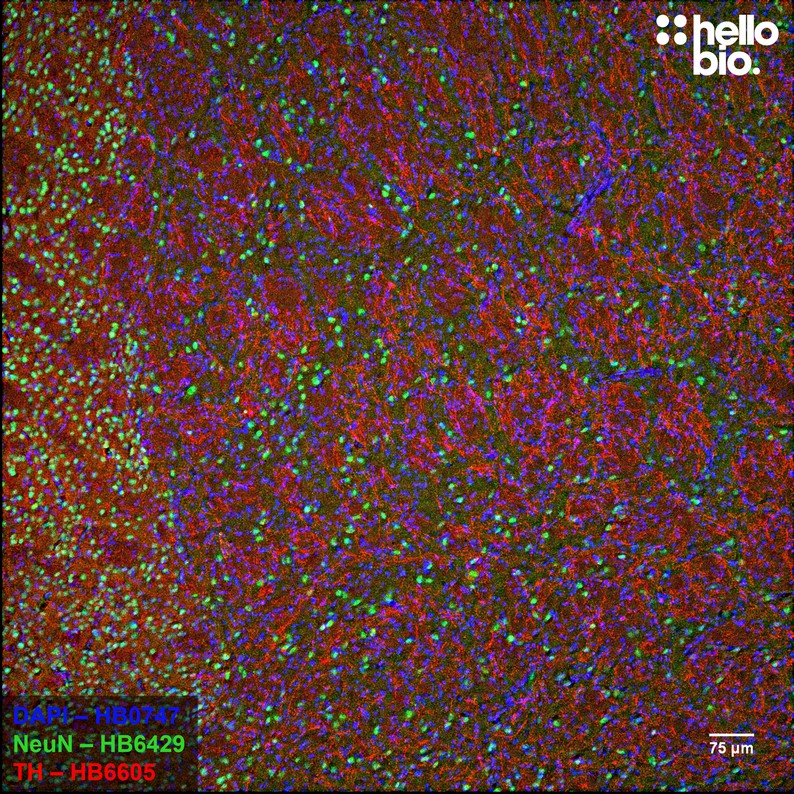
Figure 2. NeuN and Tyrosine hydroxylase expression in rat striatum.

Figure 3. Independent antibody validation of HB6429 in rat cortex.
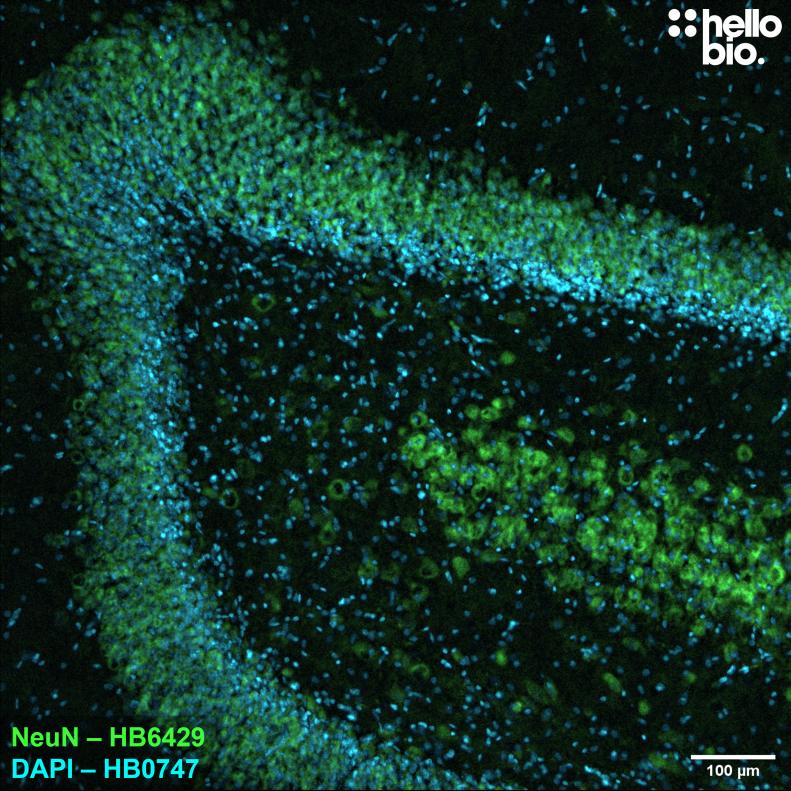
Figure 4. NeuN expression in the rat dentate gyrus visualised using HB6429.

Figure 5. NeuN expression in the granule cell layer of the rat dentate gyrus visualised using HB6429.

Figure 6. The effect of varying HB6429 concentration upon staining in rat dentate gyrus
HB6429 successfully labelled the dense layer of neurones present in the granule cell layer of the dentate gyrus in 40µm rat hippocampal sections at a range of dilutions. Method: hippocampi were dissected from rat brains and fixed overnight in 4% PFA before then being incubated in 30% sucrose (in PBS) for another 24hrs. A freezing microtome was used to cut 40µm transverse slices before sections were incubated in 0.05M glycine for 30 minutes. Sections were blocked in 1% BSA, 22.52mg/ml glycine before incubation overnight in varying concentrations of HB6429 (1:500 to 1:4,000 dilutions, 0.25-2µg/ml). This was followed by a two hour incubation with secondary antibody (Polyclonal goat anti-mouse DyLight 488 conjugated, Thermofisher 35503, 1:300 dilution). DAPI (HB0747) was used at 1µg/ml to visualise cell nuclei (not shown due to obscuring NeuN signal in the dense cell layer). For more detail please see our IHC(IF) protocol. Images were captured using a Leica DM2500 epifluorescence microscope (20x objective) coupled to a Leica DFC7000T colour digital camera with DAPI and I3 filters. Exposure times were as follows:
- 1:500 - I3: 10x gain, 32.2ms exposure
- 1:1000 - I3: 10x gain, 10x gain, 22.3ms exposure
- 1:2000 – I3: 10x gain, 22.3ms exposure
- 1:4000 – I3: 10x gain, 17.9ms exposure
- No primary - I3: 112.ms exposure (taken using different microscope: Leica DMI6000B with Photometric-Prime95B camera).

Figure 7. NeuN expression in various tissue lysates and preparations.
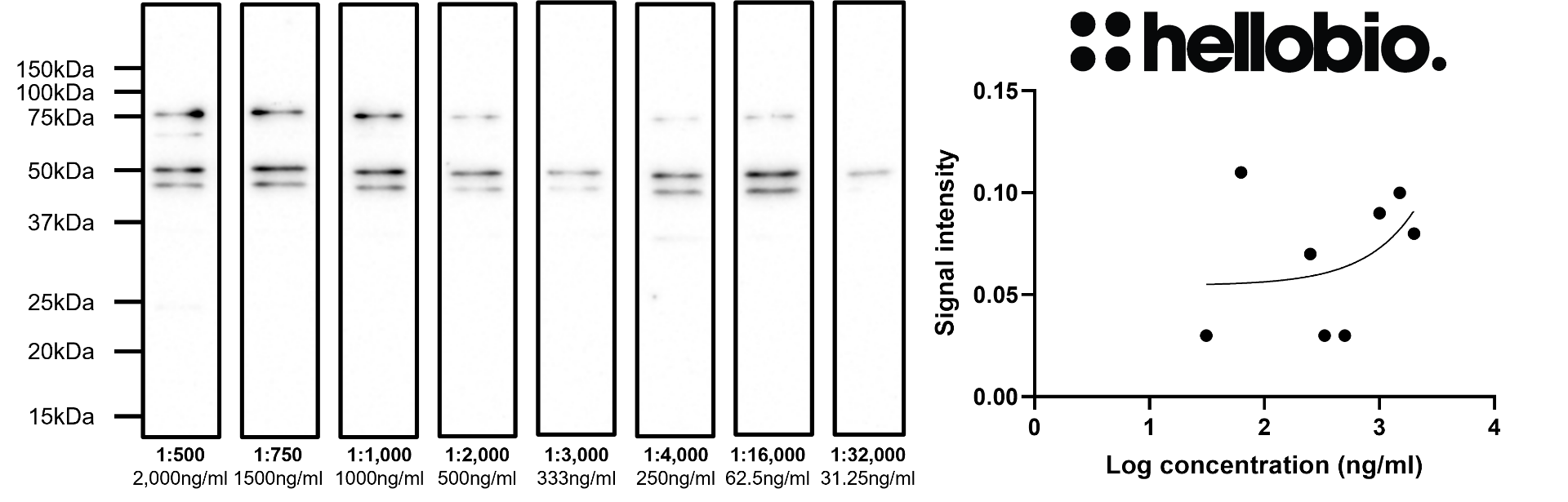
Figure 8. Concentration response of HB6429 staining in rat brain cytosol preparation.

Figure 9. NeuN expression in various tissue lysates and preparations including GAPDH (HB9177) loading control.
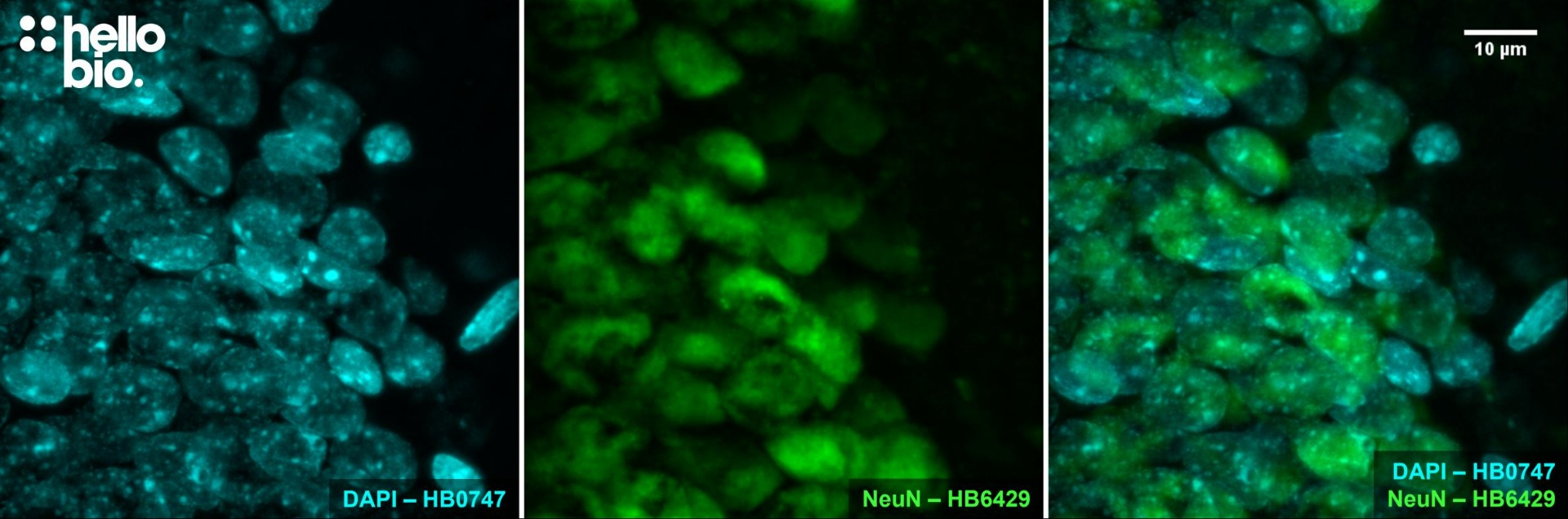
Figure 10. NeuN expression in the granule cell layer of the rat dentate gyrus visualised using HB6429.
HB6429 successfully stains the dense layer of neurones present in the rat dentate gyrus in PFA fixed sections. Method: hippocampi were dissected from rat brains and fixed overnight in 4% PFA before then being incubated in 30% sucrose (in PBS) for another 24hrs. A freezing microtome was used to cut 40µm transverse slices before sections were incubated in 0.05M glycine for 30 minutes. Sections were blocked in 1% BSA, 22.52mg/ml glycine before incubation overnight in HB6429 (1:1,000 dilution, 1µg/ml). This was followed by a two hour incubation with secondary antibody (Polyclonal goat anti-mouse DyLight 488 conjugated, Thermofisher 35503, 1:300 dilution). DAPI (HB0747) was used at 1µg/ml to visualise cell nuclei. For more detail please see our IHC(IF) protocol. The image was captured using a Leica SPE confocal laser scanning microscope coupled to a Leica DMi8 inverted epifluorescence microscope. The image was captured using a 40x objective (5.5x zoom) in a z-stack (168nm spacing) and 405 (20.0% power, gain: 494) / 488 (27.0% power, gain: 619) laser lines. The image was processed in ImageJ (Schindelin et al., 2012. Nat Methods, 9(7), 676–682).
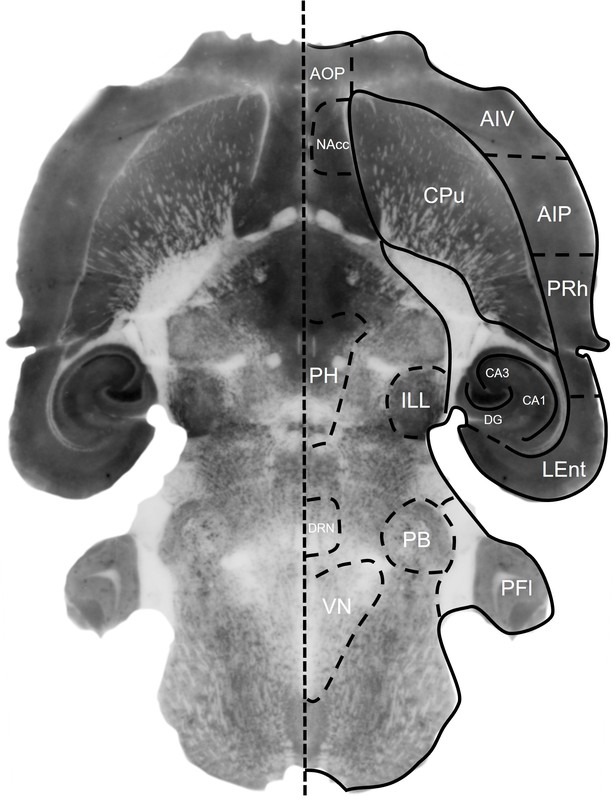
Figure 11. NeuN histoblot in a horizontal rat brain section using HB6429.
HB6429 reveals the distribution of NeuN expressing neurons within the rat brain using histoblotting. Note image has been mirrored to show annotated brain regions. Abbreviations: AIV: Agranular insular cortex (ventral), AOP: Anterior olfactory area (posterior), AIP: Agranular insular cortex (posterior), CA1: Hippocampal CA1, CA3: Hippocampal CA3, Cpu: Caudate Putamen, DG: Dentate gyrus, DRN: Dorsal raphe nucleus, ILL: Intermediate nucleus of the lateral lemniscus, Lent: Lateral entorhinal cortex, Nacc: Nucleus accumbens, PB: Parabrachial nucleus, PFl: Paraflocculus PH: Posterior hypothalamic nucleus, PRh: Perirhinal cortex, VN: Vestibularn nucleus. Method: Histoblots were performed following the methodology detailed in Molar, 2016. Neuromethods). In brief: 10µm fresh frozen sections were prepared on a cryostat from rat brains and transferred from slides to nitrocellulose membranes. Membranes were incubated in DNase I for 20 minutes at 37°C before incubation in stripping buffer containing β-mercaptoethanol for 1hr at 45°C. Sections were then blocked in 5% non-fat dry milk in TBST for 1hr before being incubated in HB6429 at 1µg/ml (1:1000 dilution) overnight at 4°C. Following washing, membranes were incubated in secondary antibody (goat anti-mouse alkaline phosphatase conjugated, Thermofisher A16069) at a 1:5,000 dilution for 90 minutes at room temperature. Membranes were developed using HB1881 NBT/BCIP solution before colour development was stopped with PBS. Membranes were imaged on a high-resolution desktop scanner.
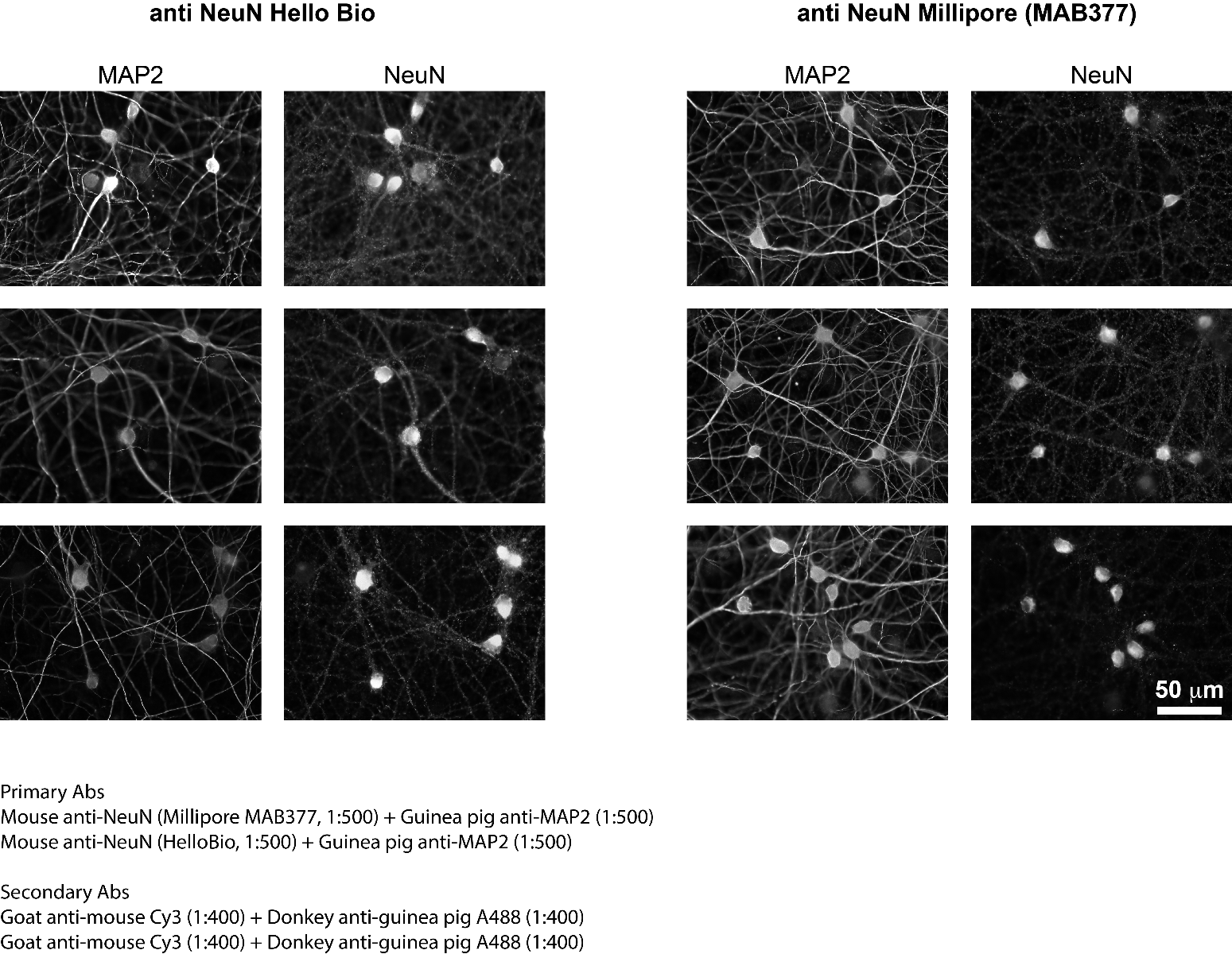
Immunostainings comparing NeuN staining in mouse brain slices













Figure 1. HB6429 staining of NeuN expressing neurons in rat hippocampus.

Figure 2. NeuN and Tyrosine hydroxylase expression in rat striatum.

Figure 3. Independent antibody validation of HB6429 in rat cortex.

Figure 4. NeuN expression in the rat dentate gyrus visualised using HB6429.

Figure 5. NeuN expression in the granule cell layer of the rat dentate gyrus visualised using HB6429.

Figure 6. The effect of varying HB6429 concentration upon staining in rat dentate gyrus
HB6429 successfully labelled the dense layer of neurones present in the granule cell layer of the dentate gyrus in 40µm rat hippocampal sections at a range of dilutions. Method: hippocampi were dissected from rat brains and fixed overnight in 4% PFA before then being incubated in 30% sucrose (in PBS) for another 24hrs. A freezing microtome was used to cut 40µm transverse slices before sections were incubated in 0.05M glycine for 30 minutes. Sections were blocked in 1% BSA, 22.52mg/ml glycine before incubation overnight in varying concentrations of HB6429 (1:500 to 1:4,000 dilutions, 0.25-2µg/ml). This was followed by a two hour incubation with secondary antibody (Polyclonal goat anti-mouse DyLight 488 conjugated, Thermofisher 35503, 1:300 dilution). DAPI (HB0747) was used at 1µg/ml to visualise cell nuclei (not shown due to obscuring NeuN signal in the dense cell layer). For more detail please see our IHC(IF) protocol. Images were captured using a Leica DM2500 epifluorescence microscope (20x objective) coupled to a Leica DFC7000T colour digital camera with DAPI and I3 filters. Exposure times were as follows:
- 1:500 - I3: 10x gain, 32.2ms exposure
- 1:1000 - I3: 10x gain, 10x gain, 22.3ms exposure
- 1:2000 – I3: 10x gain, 22.3ms exposure
- 1:4000 – I3: 10x gain, 17.9ms exposure
- No primary - I3: 112.ms exposure (taken using different microscope: Leica DMI6000B with Photometric-Prime95B camera).

Figure 7. NeuN expression in various tissue lysates and preparations.

Figure 8. Concentration response of HB6429 staining in rat brain cytosol preparation.

Figure 9. NeuN expression in various tissue lysates and preparations including GAPDH (HB9177) loading control.

Figure 10. NeuN expression in the granule cell layer of the rat dentate gyrus visualised using HB6429.
HB6429 successfully stains the dense layer of neurones present in the rat dentate gyrus in PFA fixed sections. Method: hippocampi were dissected from rat brains and fixed overnight in 4% PFA before then being incubated in 30% sucrose (in PBS) for another 24hrs. A freezing microtome was used to cut 40µm transverse slices before sections were incubated in 0.05M glycine for 30 minutes. Sections were blocked in 1% BSA, 22.52mg/ml glycine before incubation overnight in HB6429 (1:1,000 dilution, 1µg/ml). This was followed by a two hour incubation with secondary antibody (Polyclonal goat anti-mouse DyLight 488 conjugated, Thermofisher 35503, 1:300 dilution). DAPI (HB0747) was used at 1µg/ml to visualise cell nuclei. For more detail please see our IHC(IF) protocol. The image was captured using a Leica SPE confocal laser scanning microscope coupled to a Leica DMi8 inverted epifluorescence microscope. The image was captured using a 40x objective (5.5x zoom) in a z-stack (168nm spacing) and 405 (20.0% power, gain: 494) / 488 (27.0% power, gain: 619) laser lines. The image was processed in ImageJ (Schindelin et al., 2012. Nat Methods, 9(7), 676–682).

Figure 11. NeuN histoblot in a horizontal rat brain section using HB6429.
HB6429 reveals the distribution of NeuN expressing neurons within the rat brain using histoblotting. Note image has been mirrored to show annotated brain regions. Abbreviations: AIV: Agranular insular cortex (ventral), AOP: Anterior olfactory area (posterior), AIP: Agranular insular cortex (posterior), CA1: Hippocampal CA1, CA3: Hippocampal CA3, Cpu: Caudate Putamen, DG: Dentate gyrus, DRN: Dorsal raphe nucleus, ILL: Intermediate nucleus of the lateral lemniscus, Lent: Lateral entorhinal cortex, Nacc: Nucleus accumbens, PB: Parabrachial nucleus, PFl: Paraflocculus PH: Posterior hypothalamic nucleus, PRh: Perirhinal cortex, VN: Vestibularn nucleus. Method: Histoblots were performed following the methodology detailed in Molar, 2016. Neuromethods). In brief: 10µm fresh frozen sections were prepared on a cryostat from rat brains and transferred from slides to nitrocellulose membranes. Membranes were incubated in DNase I for 20 minutes at 37°C before incubation in stripping buffer containing β-mercaptoethanol for 1hr at 45°C. Sections were then blocked in 5% non-fat dry milk in TBST for 1hr before being incubated in HB6429 at 1µg/ml (1:1000 dilution) overnight at 4°C. Following washing, membranes were incubated in secondary antibody (goat anti-mouse alkaline phosphatase conjugated, Thermofisher A16069) at a 1:5,000 dilution for 90 minutes at room temperature. Membranes were developed using HB1881 NBT/BCIP solution before colour development was stopped with PBS. Membranes were imaged on a high-resolution desktop scanner.

Immunostainings comparing NeuN staining in mouse brain slices












Product information
| Immunogen | Amino acids 213 - 310 of human FOX3 expressed and purified from E. coli |
| Clone number | 1B7 |
| Isotype | IgG2b |
| Purification | Protein G affinity chromatography |
| Concentration | 1mg/ml |
| Formulation | 50% PBS, 50% glycerol + 5mM sodium azide |
| Predicted species reactivity | Human, Mouse, Rat |
| Tested species reactivity | Mouse, Rat |
Tested applications
| Applications | WB, IHC(IF), Histoblot |
| Western blot optimal concentration | 1:1000 (1μg/ml) as assessed in a rat brain cytosol preparation |
| IHC(IF) optimal concentration | 1:1000 (1μg/ml) as assessed in rat hippocampal sections |
| ICC optimal concentration | 1:1000 (1μg/ml) as assessed in rat horizontal brain sections |
| Positive control | NeuN is highly expressed in the neurons of the CNS and PNS. It is also expressed in SH-SY5Y cells. |
| Negative control | Any tissue not of neural origin. Most cell lines are NeuN negative. |
| Open data link | Please follow this link to OSF |
Target information
| Other names | FOX3, RNA binding protein fox-1 homolog 3, Fox-1 homolog C, RBFOX3, RFOX3 |
| UniProt ID | A6NFN3 |
| Structure image | 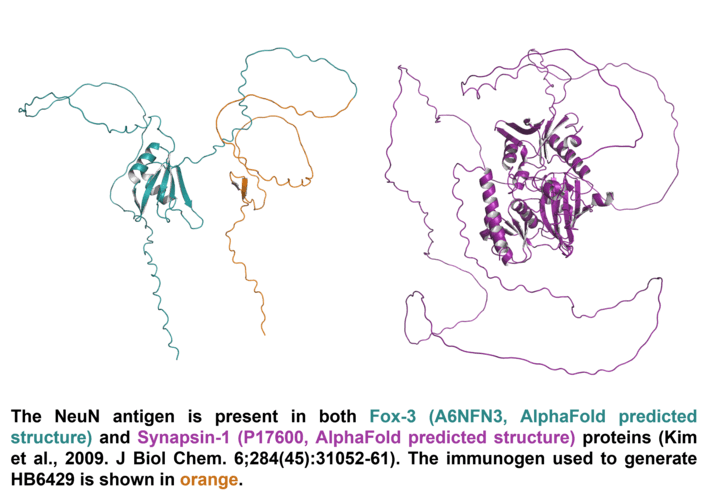 |
| Gene name | RBFOX3 |
| NCBI full gene name | RNA binding fox-1 homolog 3 |
| Entrez gene ID | 146713 |
| Amino acids | Dependent on isoform |
| Isoforms | NeuN binds primarily to FOX3 which has two isoforms. Isoform 1 is described as the canonical sequence with 312 amino acids (33.8kDa) while isoform 2 has a 13 residue insert at position 312 leading to a total length of 325 amino acids (35.1kDa). NeuN antibodies also bind to synapsin-1 in western blot experiments (but not in IHC or ICC) which has two isoforms. Isoform 1 is 705aa long (74.1kDa) while isoform 2 is shorter at 669aa (70.0kDa). |
| Expression | NeuN is expressed only within neurones. While the vast majority of neurones express NeuN some cell types such as Purkinje cells, stellate and golgi cells do not show immunoreactivity. |
| Subcellular expression | Expression is primarily localised to the nucleus however some FOX3 isoforms can localise to the cytosol. |
| Target function | FOX3 is a splicing regulator of pre-mRNA responsible for neuronal specific alternative splicing of neuronal proteins. |
| Processing | None |
| Post translational modifications | Phosphorylation has been reported (see Lind et al., 2004. J Neurosci Res. 79: 295-302) which is directly related to immunoreactivity whereby dephosphorylation abolished staining. |
| Homology (compared to human) | Mouse FOX3 shows 95.02% identity to human FOX3 wheras rat FOX3 shows no similarity due to a large 47 residue insertion at amino acid 252 in rats. |
| Similar proteins | RNA-binding protein fox-1 homolog 1 (40-44kDa) shows 67.3% identity while RNA-binding protein fox-1 homolog 2 (37-47kDa) shows 56.5% identity |
Storage & Handling
| Storage instructions | -20°C |
| Shipping Conditions | On ice |
| Important | This product is for RESEARCH USE ONLY and is not intended for therapeutic or diagnostic use. Not for human or veterinary use |
Technical guides
FAQs
NeuN is a widely used marker for identifying neurons in the central nervous system. It is a nuclear and sometimes perinuclear protein expressed in the majority of post-mitotic (mature) neurons, making it highly specific for neuronal identification.
We recommend using one of our high performance mounting medias, supplied as either hardset or aqeous with a range of counterstains:
Hardset:
- HB6966 - MightyMountTM Antifade Fluorescence Mounting Medium (hardset)
- HB8459 - MightyMountTM Antifade Fluorescence Mounting Medium with DAPI (hardset)
- HB7033 - MightyMountTM Antifade Fluorescence Mounting Medium with Propidium Iodide (hardset)
- HB7508 - MightyMountTM Antifade Fluorescence Mounting Medium with Phalloidin-TRITC (hardset)
Aqueous:
- HB9854 - MightyMountTM Antifade Fluorescence Mounting Medium (aqueous)
- HB7618 - MightyMountTM Antifade Fluorescence Mounting Medium with DAPI (aqueous)
- HB8761 - MightyMountTM Antifade Fluorescence Mounting Medium with Propidium Iodide (aqueous)
- HB9417 - MightyMountTM Antifade Fluorescence Mounting Medium with Phalloidin-TRITC (aqueous)
Please see our guide to choosing the right neuronal and astrocyte markers.
While the vast majority of neurones express NeuN some subtypes such as Purkinje cells, stellate and golgi cells do not show immunoreactivity. NeuN is also not expressed in immature neurones.
We guarantee that your NeuN antibody will work for the applications and species we list on the datasheet. If the antibody fails to perform as expected then we are happy to offer a 100% refund guarantee. For more details please see our guarantee policy.
A species not being listed doesn’t mean that the antibody won’t work, just that we haven’t tested it. If you test one of our antibodies in a new species please let us know (positive or negative)!
We have made a comprehensive collection of protocols that we have used in our experiments to validate this NeuN antibody.
We recommend using either DAPI or Hoechst 33342 to label cell nuclei. In some experiments it is also helpful to label actin filaments in the cytoskeleton using a Phalloidin conjugate such as FITC Phalloidin or Rhodamine Phalloidin-TRITC.
For any other questions about our antibody products please see our technical FAQs for antibodies
References for Anti-NeuN antibody ValidAb™
-
Novel Insights into NeuN: from Neuronal Marker to Splicing Regulator
Duan W et al (2016) Molecular neurobiology 53(3) : 1637–1647 -
NeuN As a Neuronal Nuclear Antigen and Neuron Differentiation Marker
Gusel'nikova VV et al (2015) Acta Naturae 7(2) : 42-7 -
Identification of neuronal nuclei (NeuN) as Fox-3, a new member of the Fox-1 gene family of splicing factors
Kim KK et al (2009) Biological Chemistry 284(45) : 31052-61 -
Characterization of the neuronal marker NeuN as a multiply phosphorylated antigen with discrete subcellular localization
Lind D et al (2005) Journal of Neuroscience Research 79(3) : 295-302 -
NeuN: a useful neuronal marker for diagnostic histopathology
Wolf et al (1996) Journal of histochemistry and cytochemistry 44(10) : 1167–1171
-
Inhibitory actions of melanin-concentrating hormone in the lateral septum.
Payant MA et al (2024) The Journal of physiologyPubMedID: 38874572 -
Cellular and Functional Role of Melanin-Concentrating Hormone in the Lateral Septum
Payant M (2024) Thesis https://doi.org/10.22215/etd/2024-15908
2 Item(s)
Antibody to NeuN - marker for mature neurones expressed in the nucleus. Part of the ValidAb™ range of highly validated, data-rich antibodies.
Standard: $35
Hazardous: $35
Shipped on ice: $35
Shipping will be calculated at checkout
Call 609-683-7500
Fax 609-228-4994
Related Products
- Code:
- HB9177
Antibody to GAPDH - universal loading control for western blotting. Part of the ValidAb™ range of highly validated, data-rich antibodies.
- Code:
- HB6433
Antibody to Neurofilament L - neurofilament component expressed in neurones. Part of the ValidAb™ range of highly validated, data-rich antibodies.
- Code:
- HB8267
Antibody to GFAP - cytoskeletal protein used as an astrocyte marker. Part of the ValidAb™ range of highly validated, data-rich antibodies.





 Paraffin Embedded IHC Protocol (IHC-P)
Paraffin Embedded IHC Protocol (IHC-P)