Anti-GAPDH antibody ValidAbTM
Certificate of Analysis
Product overview
| Name | Anti-GAPDH antibody ValidAbTM |
| Host | Mouse |
| Clonality | Monoclonal |
| Target | GAPDH |
| Description | Antibody to GAPDH - universal loading control for western blotting. Part of the ValidAb™ range of highly validated, data-rich antibodies. |
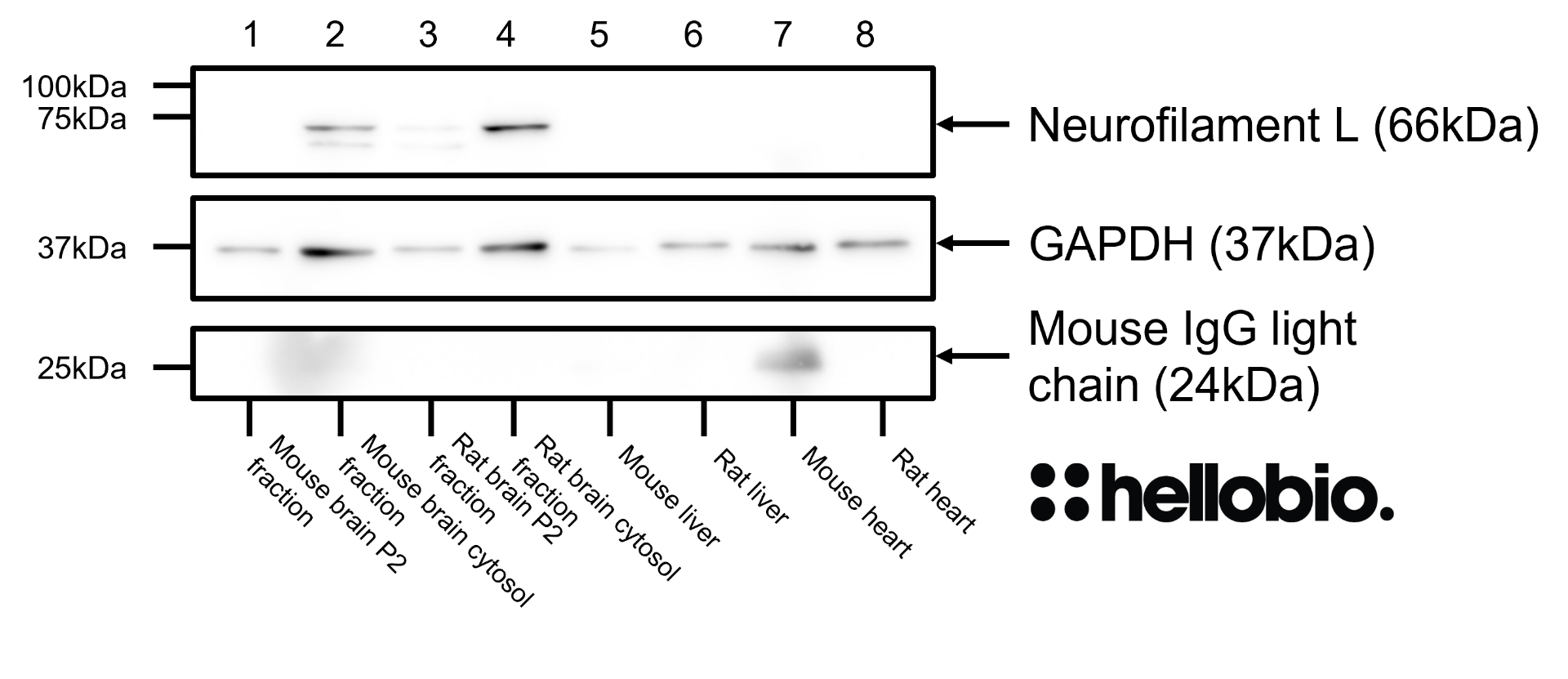
Validation data
Product information
| Immunogen | Purified rabbit GAPDH |
| Clone number | 6C5cc |
| Isotype | IgG1 |
| Purification | Protein A affinity chromatography |
| Concentration | 1mg/ml |
| Formulation | Lyophilised. When reconstituted contains PBS with 0.09% sodium azide and 1% recombinant albumin |
| Predicted species reactivity | Mouse, Rat, Human, Pig, Dog, Rabbit, Cat, Fish |
| Tested species reactivity | Mouse, Rat, Human |
Tested applications
| Applications | ELISA, ICC, WB |
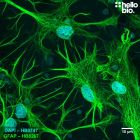
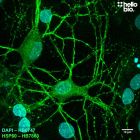
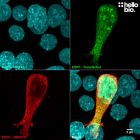
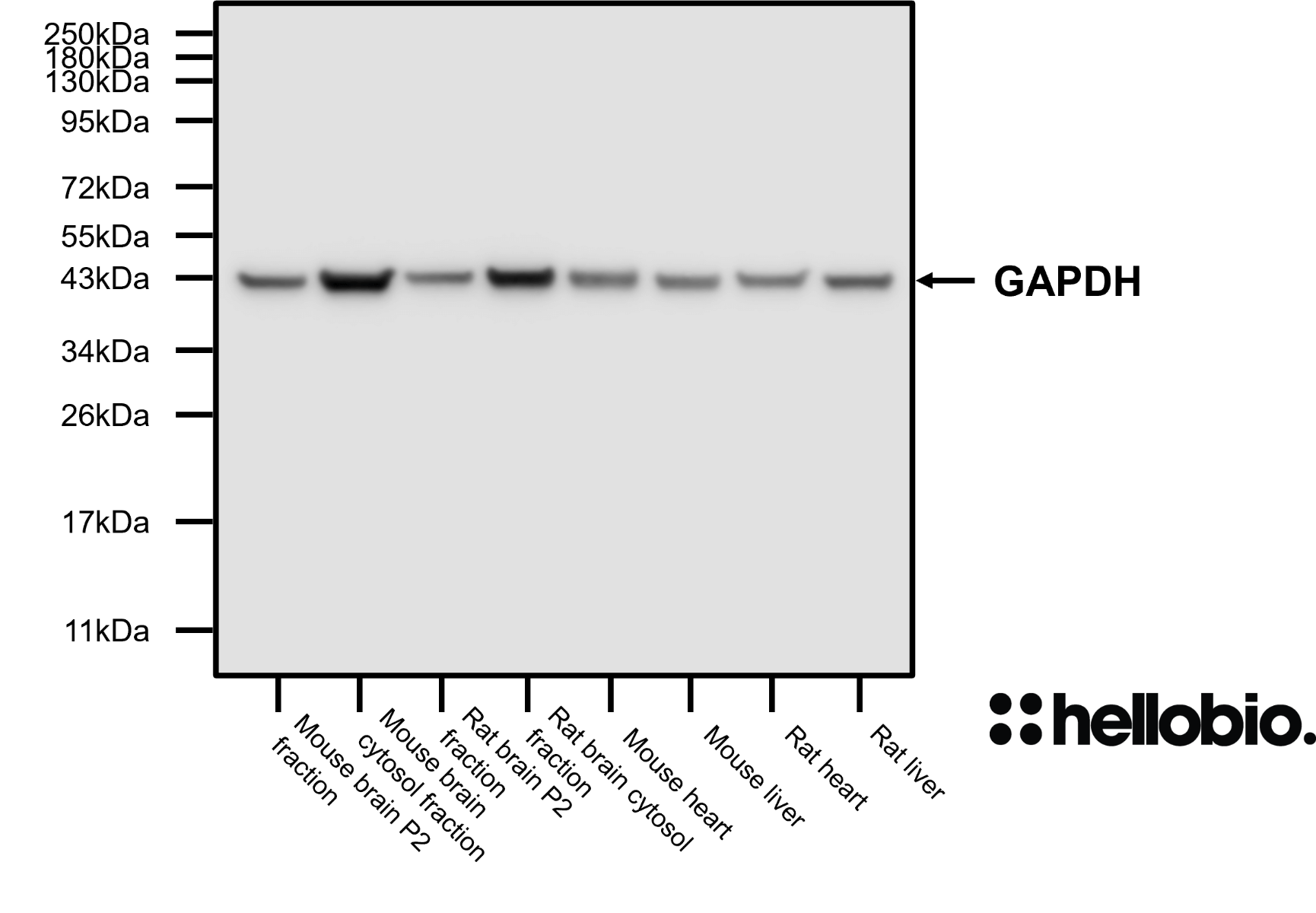
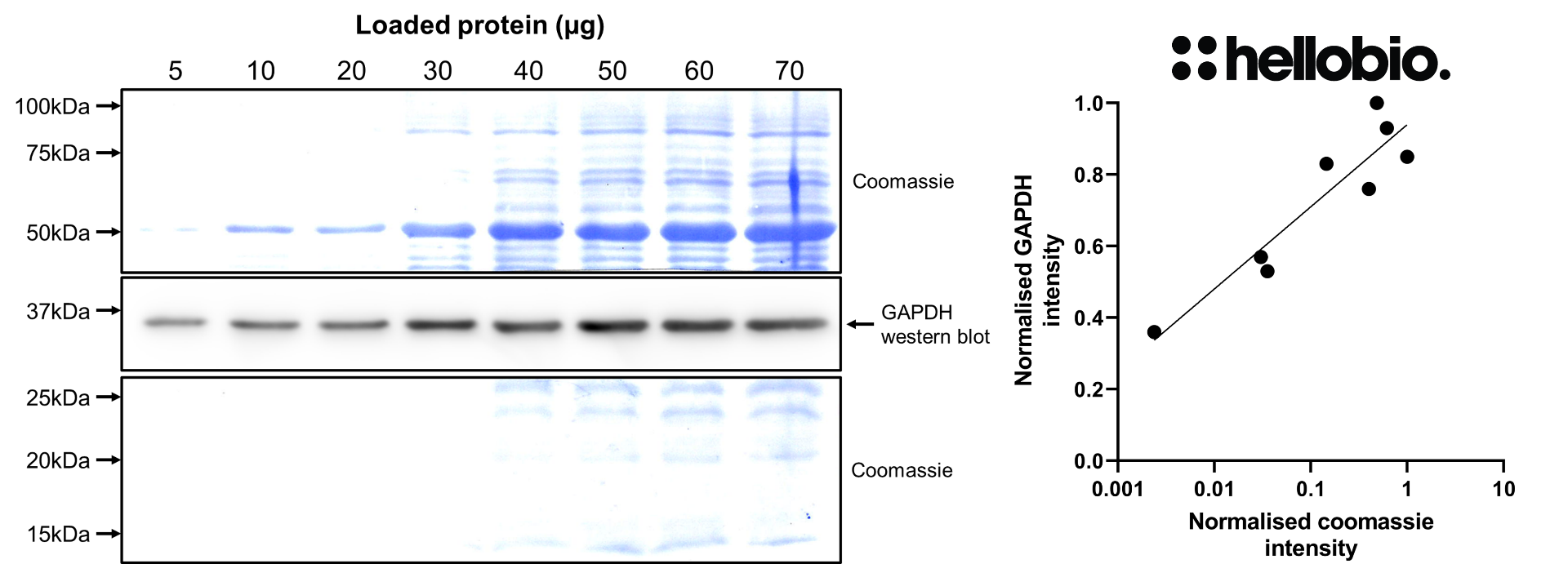
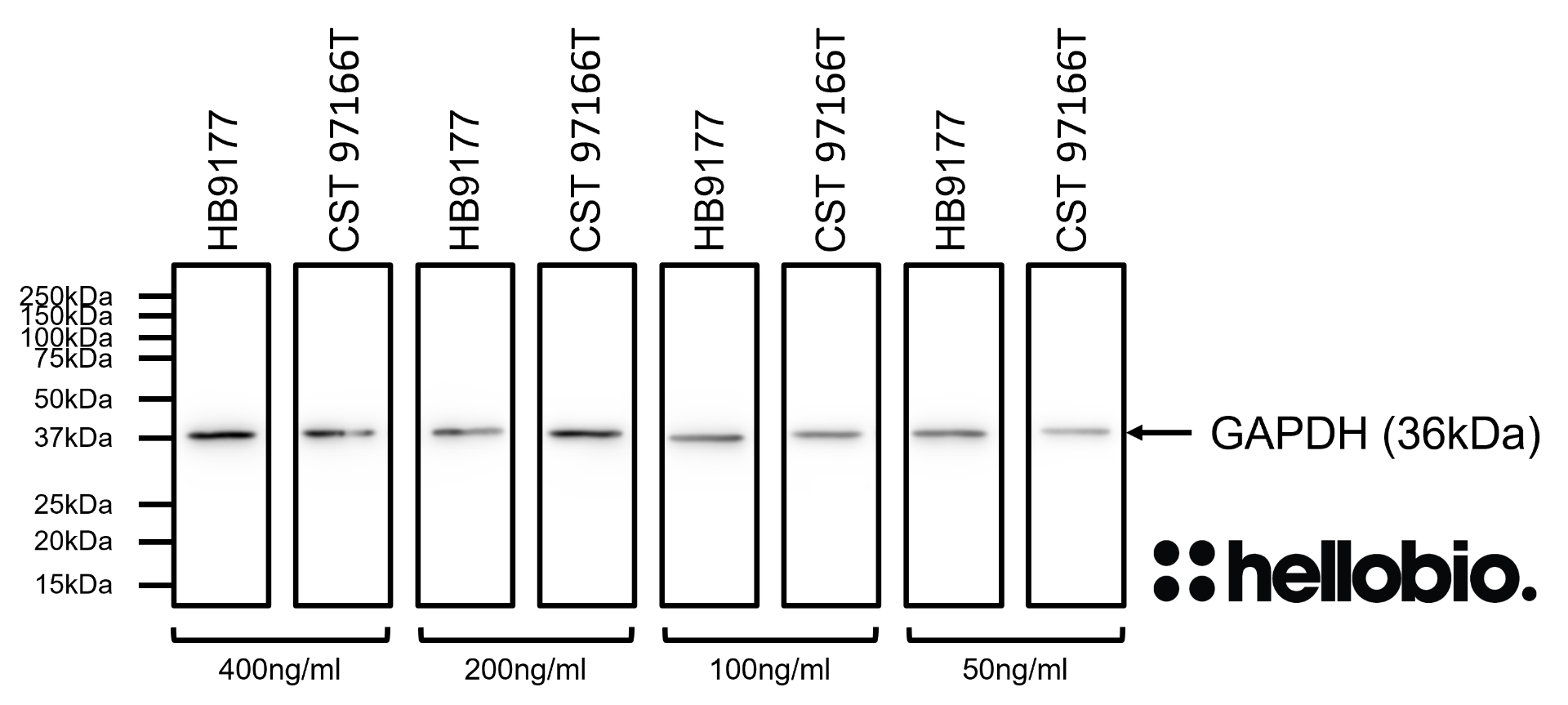
| Western blot optimal concentration | 0.25μg/ml (1:4,000) as measured in rat brain cytosol preparation |
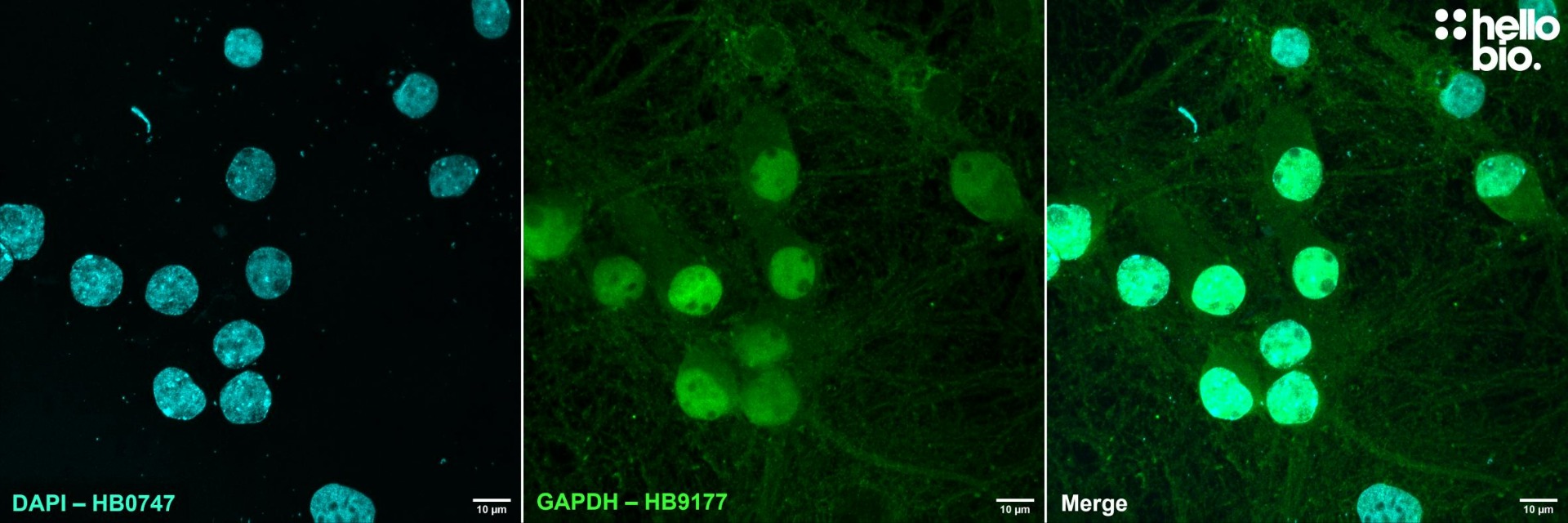
| ICC optimal concentration | 2μg/ml (1:500) as measured in cultured rat neurones |
| Positive control | GAPDH is ubiquitously expressed at high levels in nearly all mammalian tissues and cells. It is also widely expressed in common cell lines. |
| Negative control | GAPDH is a cytosolic enzyme, so complete subcellular fractionation should be sufficient to provide a negative control. Due to its high expression, care should be taken to ensure that fractionation is complete without any cytosolic contamination. |
| Open data link | Please follow this link to OSF |
Target information
| Other names | Glyceraldehyde-3-phosphate dehydrogenase, GAPD, G3PD, HEL-S-162eP |
| UniProt ID | P04406 |
| Structure image | 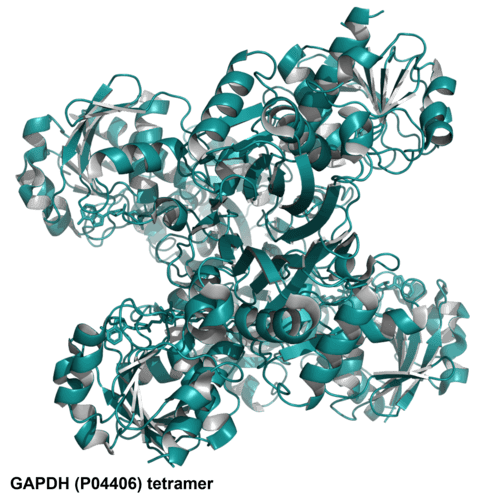 |
| Gene name | GAPDH |
| NCBI full gene name | glyceraldehyde-3-phosphate dehydrogenase |
| Entrez gene ID | 2597 |
| Amino acids | 335 (36.1kDa) |
| Isoforms | GFAP has two isoforms. Isoform 1 : 335 amino acids, 36.05kDa; Isoform 2: 293 amino acids (missing residues 1-42), 31.55kDa |
| Expression | GAPDH is expressed ubiquitously in all tissues and cell types. |
| Subcellular expression | Expression is primarily in the cytosol although there has been nuclear expression reported during high levels of cellular stress. In red blood cells GAPDH assembles on the cell membrane as part of larger multi-protein complexes. |
| Target function | GAPDH catalyses the sixth step of glycolysis therefore is essential to cellular ATP production. Other roles for GAPDH have also been revealed including transcriptional regulation of some genes and involvement in the initiation of apoptosis. |
| Processing | Following translation the leading methionine is removed to form the mature protein. |
| Post translational modifications | GAPDH is subject to numerous post-translational modifications including phosphorylation, deamination, acetylation, methylation and nitrosylation on multiple residues. |
| Homology (compared to human) | Mouse and rat show 100% homology to each other in a direct BLAST comparison while showing 99% homology to human GAPDH due humans posessing the insertion of GK at position 2. |
| Similar proteins | None |
Storage & Handling
| Storage instructions | -20°C then use reconstitution advice |
| Reconstitution advice | Upon receipt store at either -20°C or -80°C.
For 100μg packs either:
For 25μg packs either:
For more information read our guide on the best care for your product. Take care when opening as the precipitate is extremely light and can easily be lost if disturbed. When reconstituting make sure that the antibody is thoroughly dissolved by pipetting up and down before giving the antibody a brief spin at 10,000g to make sure that all material is recovered and at the bottom of the tube. |
| Shipping Conditions | Stable for ambient temperature shipping. Follow storage instructions on receipt. |
| Important | This product is for RESEARCH USE ONLY and is not intended for therapeutic or diagnostic use. Not for human or veterinary use |
References for Anti-GAPDH antibody ValidAbTM
-
An appropriate loading control for western blot analysis in animal models of myocardial ischemic infarction
Nie X et al (2017) Biochem Biophys Rep 12 : 108-113 -
Glyceraldehyde-3-phosphate dehydrogenase: a universal internal control for Western blots in prokaryotic and eukaryotic cells
Wu Y et al (2012) Analytical Biochemistry 423(1) : 15-22 -
The diverse functions of GAPDH: views from different subcellular compartments
Tristan C et al (2010) Cell Signal 23(2) : 317-323 -
S-nitrosylated GAPDH initiates apoptotic cell death by nuclear translocation following Siah1 binding
Hara M et al (2005) Nature Cell Biology 665-674 : 7(7)
-
Modulating voltage-gated sodium channels to enhance differentiation and sensitize glioblastoma cells to chemotherapy.
Giammello F et al (2024) Cell communication and signaling : CCS 22 : 434PubMedID: 39251990








 Paraffin Embedded IHC Protocol (IHC-P)
Paraffin Embedded IHC Protocol (IHC-P)