What counterstains do you recommend for use in ICC and IHC with this c-Fos antibody?
Will my c-Fos antibody work against species that have not been listed on the datasheet?
A species not being listed doesn’t mean that the c-Fos antibody won’t work, just that we haven’t tested it. If you test one of our antibodies in a new species please let us know (positive or negative)!
What protocols are available for use with this c-Fos antibody?
We have made a comprehensive collection of protocols that we have used in our experiments to validate this c-Fos antibody.
What guarantee do you have that my c-Fos antibody will perform as expected?
We guarantee that your c-Fos antibody will work for the applications and species we list on the datasheet. If the antibody fails to perform as expected then we are happy to offer a 100% refund guarantee. For more details please see our guarantee policy.
c-Fos is a transcription factor primarily used as a biomarker for cellular activation, especially in neurons, to study patterns of brain activity, learning, memory, stress, and behavior. It is also a valuable tool in cancer research, neurodegenerative disease studies, and gene expression research. c-Fos expression is widely used to map neuronal activity in response to behavioural and pharmacological manipulations.
How is c-Fos a marker of neuronal activity?
c-Fos is a protein that serves as an important marker of neuronal activity. It is a transcription factor that is produced rapidly and transiently in response to various stimuli, including neuronal activity, stress, and other cellular signals. c-Fos is an immediate early gene which is rapidly activated without the need for protein synthesis. When neurons are stimulated, for example by sensory input or other types of activity, the c-Fos gene is quickly transcribed into mRNA and translated into the c-Fos protein. This occurs within minutes after the stimulus. The expression of c-Fos is highly activity-dependent and occurs almost exclusively when neurons are actively firing or being strongly activated. Importantly, its expression is transient, meaning that the protein is produced for a short period following neuronal activation, typically peaking within 30 to 60 minutes, and then rapidly decaying. This short-lived nature makes c-Fos an ideal marker for recent neuronal activity.
What mounting media do you recommend to use with this antibody?
We recommend using one of our high performance mounting medias, supplied as either hardset or aqeous with a range of counterstains:
Hardset:
Aqueous:
What other neuroscience markers are available?






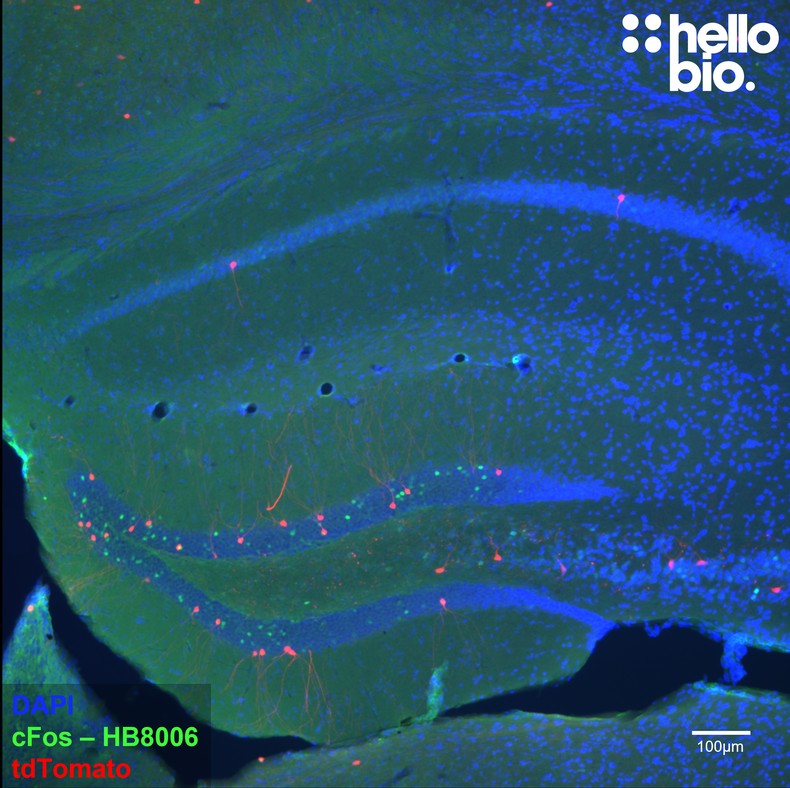
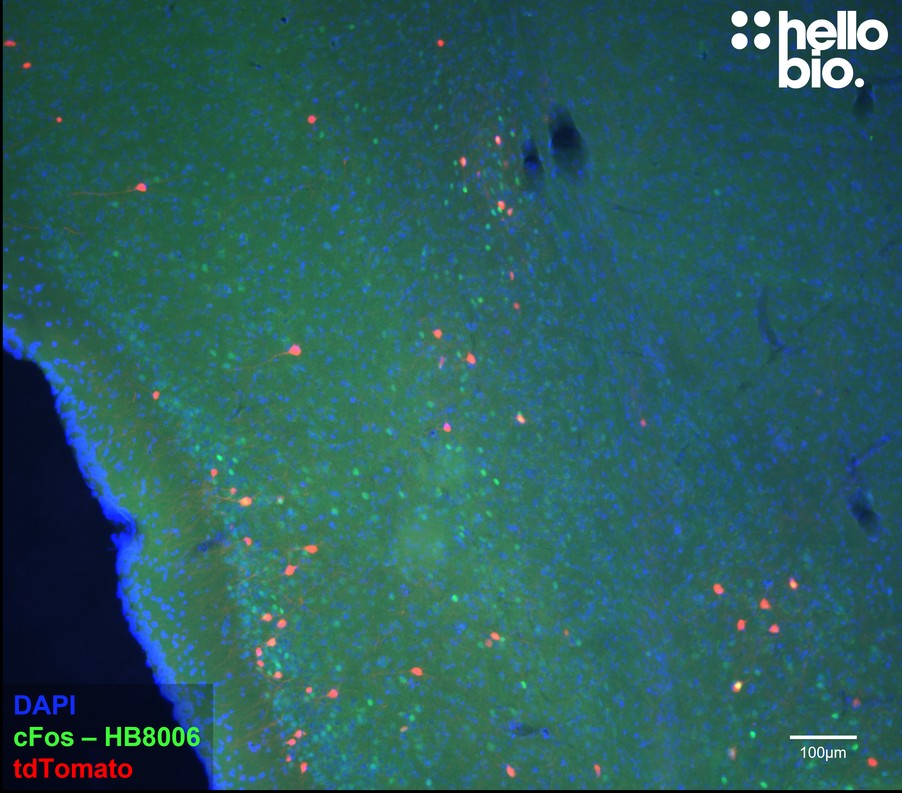
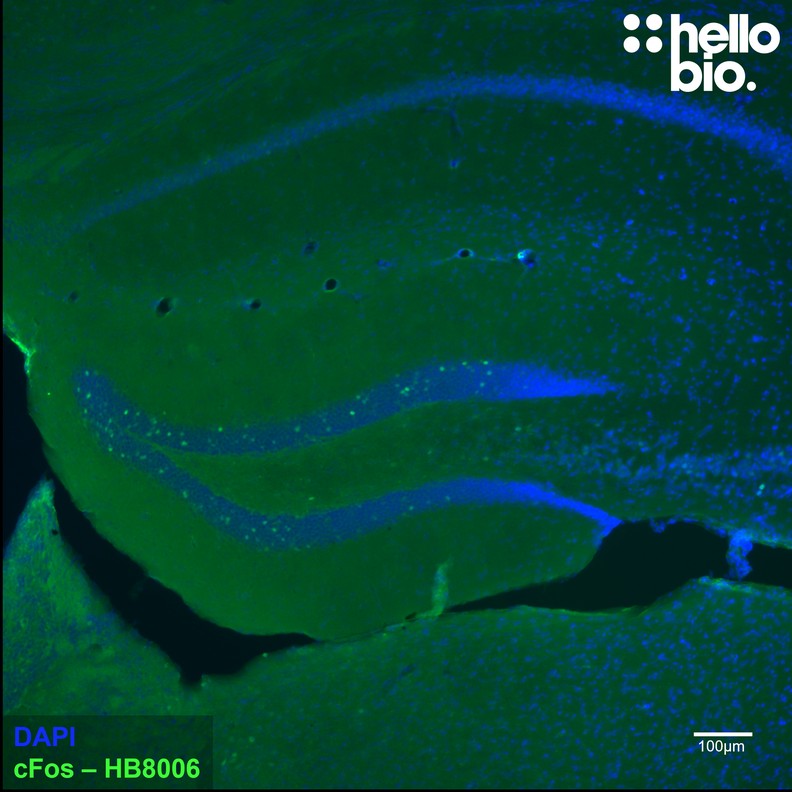
 Paraffin Embedded IHC Protocol (IHC-P)
Paraffin Embedded IHC Protocol (IHC-P)