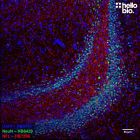
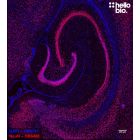
Anti-NeuN antibody ValidAb™
Certificate of Analysis
Product overview
| Name | Anti-NeuN antibody ValidAb™ |
| Alternative names | Fox-3 |
| Host | Goat |
| Clonality | Polyclonal |
| Target | NeuN |
| Description | Antibody to NeuN - marker for mature neurones expressed in the nucleus. Part of the ValidAb™ range of highly validated, data-rich antibodies. |
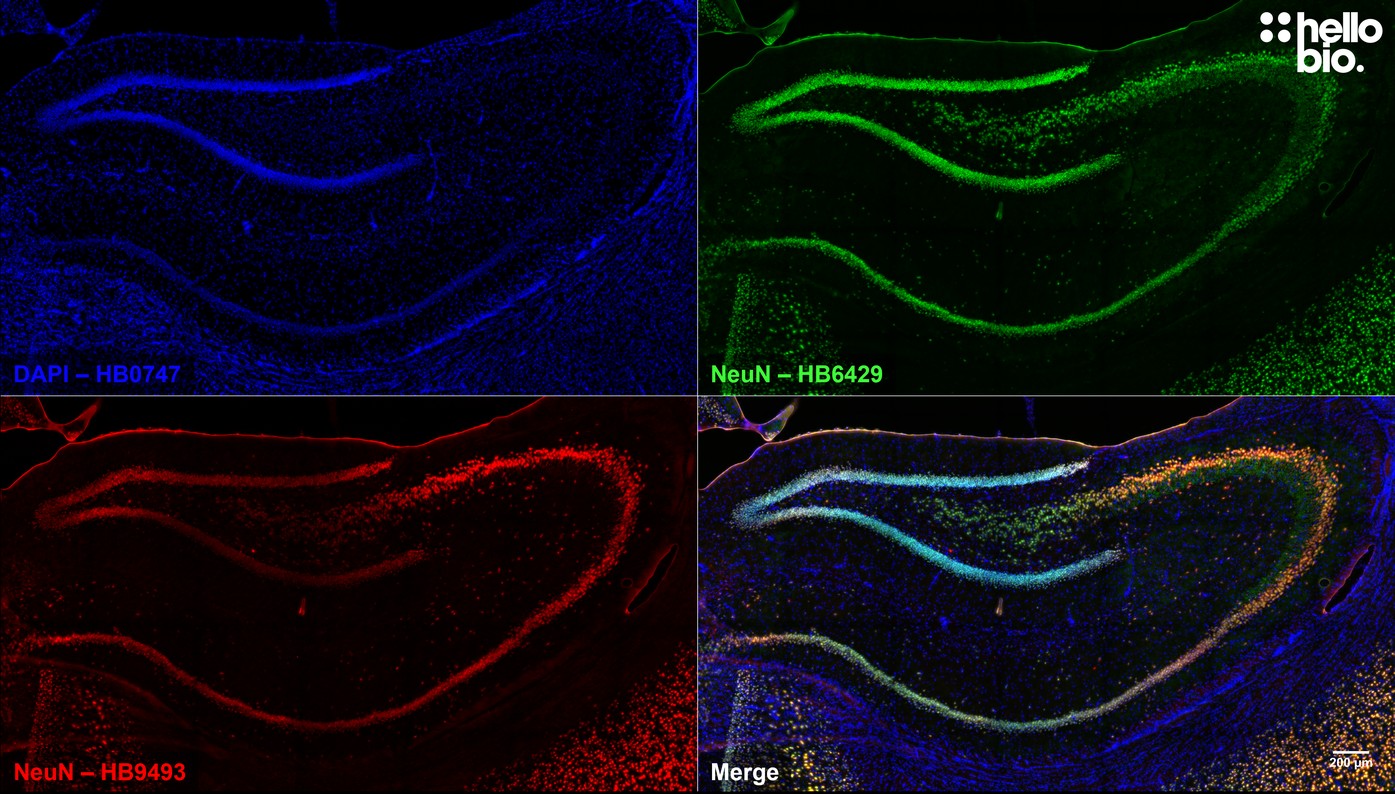
Validation data
Product information
| Immunogen | N-terminal 100 amino acids of human FOX3 expressed and purified from E. coli |
| Isotype | IgG |
| Purification | Immunogen affinity purification |
| Concentration | 1mg/ml |
| Formulation | 50% PBS, 50% glycerol + 5mM sodium azide |
| Predicted species reactivity | Mouse, Rat, Human |
| Tested species reactivity | Mouse, Rat |
Tested applications
| Applications | IHC(IF) |
| IHC(IF) optimal concentration | 1:1000 (1μg/ml) as assessed in rat brain sections |
| Positive control | NeuN is highly expressed in the neurons of the CNS and PNS. It is also expressed in SH-SY5Y cells.
|
| Negative control | Any tissue not of neural origin. Most cell lines are NeuN negative. |
| Open data link | Please follow this link to OSF. |
Target information
| Other names | FOX3, RNA binding protein fox-1 homolog 3, Fox-1 homolog C, RBFOX3, RFOX3 |
| UniProt ID | A6NFN3 |
| Structure image |  |
| Gene name | RBFOX3 |
| NCBI full gene name | RNA binding fox-1 homolog 3 |
| Entrez gene ID | 146713 |
| Amino acids | Dependent on isoform |
| Isoforms | NeuN binds primarily to FOX3 which has two isoforms. Isoform 1 is described as the canonical sequence with 312 amino acids (33.8kDa) while isoform 2 has a 13 residue insert at position 312 leading to a total length of 325 amino acids (35.1kDa). NeuN antibodies also bind to synapsin-1 in western blot experiments (but not in IHC or ICC) which has two isoforms. Isoform 1 is 705aa long (74.1kDa) while isoform 2 is shorter at 669aa (70.0kDa). |
| Expression | NeuN is expressed only within neurones. While the vast majority of neurones express NeuN some cell types such as Purkinje cells, stellate and golgi cells do not show immunoreactivity. |
| Subcellular expression | Expression is primarily localised to the nucleus however some FOX3 isoforms can localise to the cytosol. |
| Target function | FOX3 is a splicing regulator of pre-mRNA responsible for neuronal specific alternative splicing of neuronal proteins. |
| Processing | None |
| Post translational modifications | Phosphorylation has been reported (see Lind et al., 2004. J Neurosci Res. 79: 295-302) which is directly related to immunoreactivity whereby dephosphorylation abolished staining. |
| Homology (compared to human) | Mouse FOX3 shows 95.02% identity to human FOX3 wheras rat FOX3 shows no similarity due to a large 47 residue insertion at amino acid 252 in rats. |
| Similar proteins | RNA-binding protein fox-1 homolog 1 (40-44kDa) shows 67.3% identity while RNA-binding protein fox-1 homolog 2 (37-47kDa) shows 56.5% identity. |
Storage & Handling
| Storage instructions | -20°C |
| Shipping Conditions | On ice |
| Important | This product is for RESEARCH USE ONLY and is not intended for therapeutic or diagnostic use. Not for human or veterinary use |
References for Anti-NeuN antibody ValidAb™
-
Novel Insights into NeuN: from Neuronal Marker to Splicing Regulator.
Duan W et al (2016) Molecular neurobiology 53 : 1637-1647 -
NeuN As a Neuronal Nuclear Antigen and Neuron Differentiation Marker.
Gusel'nikova VV et al (2015) Acta naturae 7 : 42-7 -
Identification of neuronal nuclei (NeuN) as Fox-3, a new member of the Fox-1 gene family of splicing factors.
Kim KK et al (2009) The Journal of biological chemistry 284 : 31052-61 -
Characterization of the neuronal marker NeuN as a multiply phosphorylated antigen with discrete subcellular localization.
Lind D et al (2005) Journal of neuroscience research 79 : 295-302 -
NeuN: a useful neuronal marker for diagnostic histopathology.
Wolf HK et al (1996) The journal of histochemistry and cytochemistry : official journal of the Histochemistry Society 44 : 1167-71





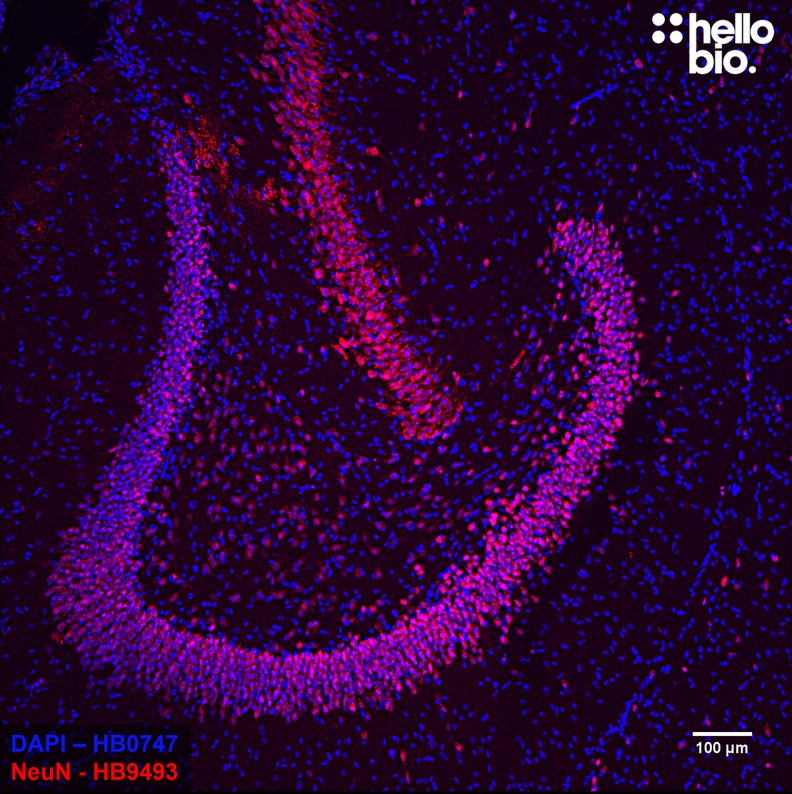
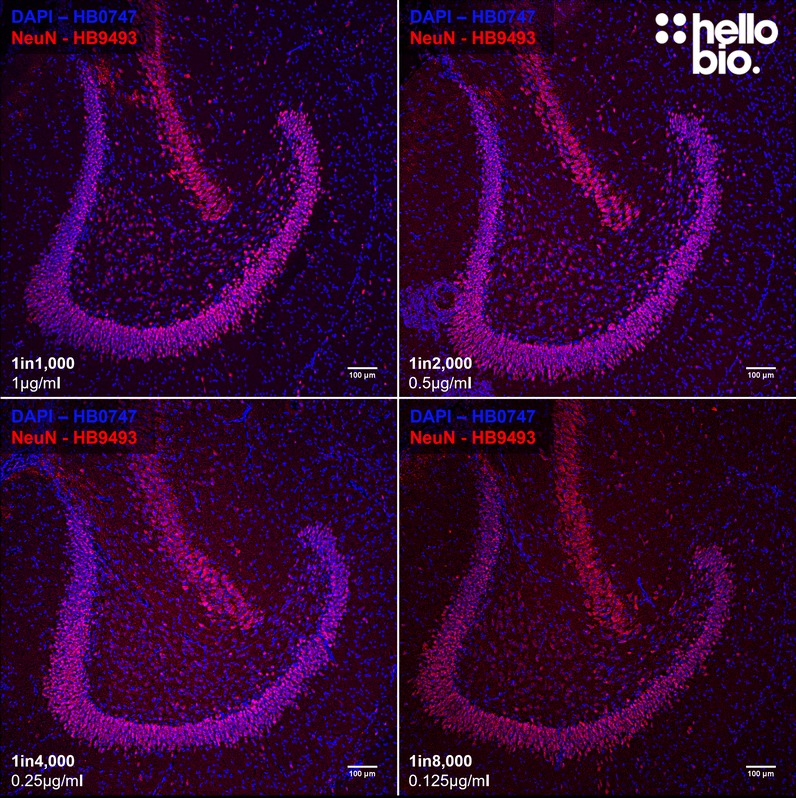
 Paraffin Embedded IHC Protocol (IHC-P)
Paraffin Embedded IHC Protocol (IHC-P)