Electrophysiology Essentials: Hands-On Learning at the Cell Physiology Workshop
For scientists looking to master the fundamentals of electrophysiology, the UK’s annual Cell Physiology Workshop is a great place to gain hands-on experience and get to grips with the essential techniques.
Founded 40 years ago by Anne Warner, David Ogden and Colin Brownlee, these intensive 2-week training courses were originally hosted by the Marine Biological Association in Plymouth, but this year found a new home at the University of Liverpool where the popular annual workshop was led by Dr Richard Rainbow.
The course attracts students and teaching staff from around the world and provides a unique practical learning experience of electrophysiological recording alongside a comprehensive series of lectures on the underlying theory and analysis of the associated data.
We asked Dr Sean Brennan, an electrophysiologist and Associate Scientist at the University of Liverpool to tell us more about the course, the techniques involved and why hands-on workshops like these are so valuable for early career life scientists.
Electrophysiology: What is it & how do scientists use it?
Most of the electrophysiology techniques taught on the Cell Physiology Workshop (CPW) are used to research the bioelectric activity of ion channels. Ion channels are tiny gateways within a cell's membrane and control the flow of ions, such as sodium, potassium, and calcium, in and out of the cell. Ion channels function like gates that open and close to help a cell send bioelectric signals and perform essential functions, like keeping your heart beating, your muscles moving, and your brain functioning correctly.
There is a lot of variety between the different types of ion channels. The gating (open and close) of each different type of ion channel can be dependent on a range of factors, such as cell excitability, temperature, and pH. The ion channels expressed in different cells (heart vs brain) are different, and the location of the ion channels within a cell can also be different. It is important to research ion channel activity from a drug safety and drug discovery perspective due to their role in key biological functions and the fact that faulty ion channel activity plays a role in several conditions such as arrhythmias and epilepsy.
All the techniques taught at CPW enable scientists to enhance our understanding of ion channel activity in health and disease. The techniques are challenging to master, so hands-on tuition like this is hugely beneficial for scientists performing these experiments.
Who is the workshop aimed at and what activities are included?
The course is primarily for UK and international early career researchers (PhD students, post-docs, scientists in industrial roles) who already have some experience in performing electrophysiology experiments who wish to receive hands-on training in performing these complex techniques. The workshop is split into three main activities:
- Lectures - The lectures cover all the key aspects of electrophysiology recordings, from constructing a recording setup, to electrophysiology recordings in various configurations and analysis of the data.
- Practical rotations - The 2-day practical rotations, which the attendees choose, provide hands-on tuition in performing electrophysiology recordings in particular configurations amongst a variety of cell and tissue types. These sessions offer an opportunity to put the theory provided in the lectures into practice.
- Interactive demonstrations - There are also interactive demonstrations of various electrophysiology techniques such as Dynamic Clamp, Multielectrode arrays, and optics.
Pictured: Students and teachers at CPW Liverpool 2024.
Primary electrophysiological techniques
The workshop covers a number of different techniques which have a variety of uses. This is not a complete list, but an overview of some of the primary techniques taught in the practical sessions in which attendees performed experiments on various high throughput electrophysiology equipment:
Manual Whole Cell Patch-Clamp
In lay terms, manual patch-clamp is a technique where a tiny hollow glass tube (the electrode) is used to connect with the membrane of a single cell to measure its bioelectrical activity. This method allows scientists to study how cells communicate through electrical signals and to measure how a drug modulates ion channel activity.
Whole Cell Patch-Clamp is arguably the most used electrophysiology technique due to its diverse range of applications. It is utilised in a variety of university laboratories to enhance our understanding of bioelectric signals, at contract research organisations performing ion channel safety screens, and in pharmaceutical companies researching potential new therapies.
This is a very popular choice for attendees, therefore two or more whole cell patch-clamp rigs are always available for the practical sessions. Attendees will perform whole-cell patch clamp experiments on cells expressing ion channels (Human embryonic kidney (HEK) or cardiomyocytes). This involves the entire technique, from pulling good electrodes to analysing the data.
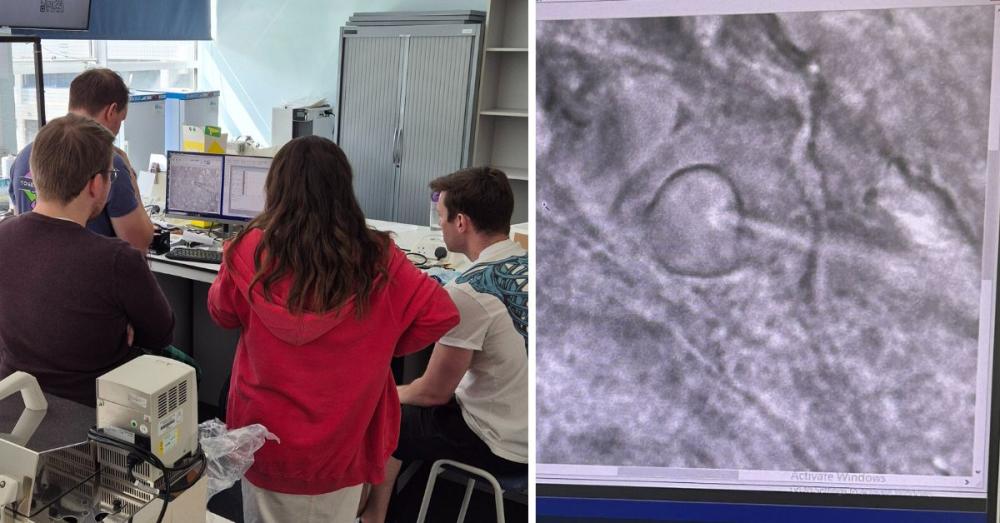
Pictured: A Whole-cell Patch-Clamp practical session at CPW 2024.
Left: Students perform manual patch clamp experiments on cardiomyocytes (top right) or HEK cells (bottom right) expressing ion channels while receiving hands-on tuition and guidance from Amy Richardson (left in blue).
Slice Electrophysiology Recording
Slice patch-clamp recordings allow researchers to observe electrical signals in a setting that closely resembles natural tissue function through maintaining the tissue's microarchitecture and some of its network connectivity. Attendees gain hands-on tuition in forming tissue slices, performing patch-clamp slice recordings, and analysis of the data.
Pictured: A slice electrophysiology practical session at CPW 2024.
Left: Gareth Morris guides the students through the process of performing a slice electrophysiology recording.
Right: An electrode is connected to a single cell within the tissue slice, which enables the students to study the bioelectric signals between adjacent cells.
Microelectrode Voltage Clamp
The workshop provides lectures and practical sessions on two electrode voltage-clamp (TEVC) and single electrode voltage clamp (SEVC). These methods use sharp glass electrodes to penetrate the cell and so are typically used on larger cells. For example, oocytes are used to model for the functional characterization of heterologously expressed ion channels. By understanding how to perform a variety of different electrophysiology configurations allows the electrophysiologist to perform the most suited experiments.
Pictured: A microelectrode voltage clamp practical session at CPW 2024, with Alasdair Gibb teaching the students the key differences between the patch-clamp technique vs microelectrode voltage clamp.
Dual Calcium Fluorescent Imaging & Patch-Clamping
In this practical, a fluorescent indicator is used to measure intracellular calcium while simultaneously measuring action potentials or ion channel currents. Attendees will learn about the range of equipment used to measure fluorescence and the different types of fluorescent dyes available. The ability to examine how drugs can modulate calcium dynamics and membrane properties means a diverse range of laboratories use this method.
Automated Patch-Clamping
Automated patch systems can perform up to 384 electrophysiology experiments in parallel. Automated patch-clamp machines are used mainly by the pharmaceutical industry/Contract Research Organisations to screen compounds against a desired ion channel target.
Automated patch clamp systems operate by positioning single cells over tiny apertures (holes) in a specialized plate with robotics and microfluidics enabling up to 348 cells to be recorded simultaneously.
Use of specialist equipment
At CPW Liverpool 2024 attendees received lectures and practical sessions from both Nanion Technologies and Sophion Biosciences, two key developers of high throughput electrophysiology equipment.
Left: Sophion Biosciences brought their QPatch semi-automated patch clamp system. Sarah Lilley taught students to perform automated patch clamp experiments on the hERG ion channel and analyse their own data using the Sophion Analyzer software.
Right: Artem Kondratskyi from Nanion Technologies brought a Port-a-patch, Orbit Mini, Orbit 16 TC and a Vesicle Prep Pro! Students were able to perform a wide range of techniques from transporter assays and planar patch clamping to bilayer recordings and giant unilamellar vesicle electroformation.
Why are workshops like CPW so valuable for scientists at PhD and postdoc level?
Attending workshops like CPW is invaluable for early career researchers. It provides both theoretical and practical training on various electrophysiology techniques, which is essential for mastering complex methodologies such as patch-clamping, fluorescence imaging, and slice electrophysiology recordings.
Through gaining hands-on tuition, attendees can understand the strengths and limitations of their own electrophysiology recordings. This insight is crucial to optimise electrophysiology recordings and to interpret the data, which improves reliability and quality of the research. In addition, the practical sessions provide plenty of opportunity for attendees to master the techniques and to ask questions about performing a recording, with hands-on guidance from experienced electrophysiologists.
Moreover, understanding the capabilities and limitations of electrophysiology equipment can also help submission for grant funding. For example, understanding the capabilities of a laser-based system vs a monochromator for fluorescence imaging can help justify the cost of new equipment and can demonstrate that it is the most cost-effective option.
Overall, workshops like this one equip early career researchers with the critical skills, knowledge, and professional connections which provide an opportunity to advance their research.
Are there opportunities for attendees to network and socialise?
Absolutely! CPW has always had an informal and collaborative atmosphere, which encourages plenty of interaction among students and instructors. There are numerous opportunities to connect, share experiences, and build relationships throughout the course.
With plenty of coffee breaks, lunches, and evening meals, the students have ample time to connect and revisit conversations. This creates a natural environment for building relationships and sharing experiences throughout the course. Typically, the last lecture will finish by 7-7.30pm, and we aim to provide attendees with accommodation that has kitchen facilities so there is the choice between eating in or exploring local restaurants. The teachers (and students if they aren’t too tired!) will often gather for an evening meal.
Pictured: Attendees gather for an evening meal after a hard day of electrophysiology, but how many PhDs does it take to order food on a smart phone?
What advice would you give to an early career scientist hoping to specialise in electrophysiology?
There are so many challenging aspects to performing electrophysiology - it will take time to master. And once you do learn, electrophysiology setups can still catch you out with the basics. For example, you may think the cells are poor quality, only to realise the air table is not floating or the temperature control is off! So, plenty of persistence and patience is required.
Gaining hands-on tuition is crucial in electrophysiology and it is the key aspect of these workshops. Early career researchers may be eligible to apply for Travel or Professional Development Awards which will fund laboratory visits or attendance to training workshops, such as CPW, to learn specific techniques.
How can readers apply for CPW Liverpool 2025?
CPW Liverpool 2025 is likely to commence between 11th – 22nd August 2025, and will advertise for applications to attend early next year between February and March 2025. Be sure to follow @CPW_Liverpool on X (Twitter) for updates.
Any tips on applying for the next workshop?
CPW is a very enjoyable course, but it is an intensive course because there are a lot of challenging topics to cover. For this reason, applicants will gain more from the workshop with some previous electrophysiology experience. Experience does not mean peer-review publication(s), just some experience performing electrophysiology recordings. In your application, briefly describe your electrophysiology experience and your intentions to perform electrophysiology recordings in the future. Do not be discouraged if your application is not successful as there are a lot of applicants each year and please do try again.
Acknowledgements
Between 1984 to 2023, over 600 students attended the Plymouth Cell Physiology Workshop to gain hands-on tuition in performing electrophysiology recordings. This workshop would not have been possible without the organisers David Ogden and Colin Brownlee.
Pictured: Students and staff from CPW 1998, MBA, Plymouth, including (top and bottom left) organisers David Ogden and Colin Brownlee.
Teachers who have contributed to CPW in recent years
B. Amos, B. Barbour, D. Bell, M. Beato, G. Bhumbra, A. Blot, J. Bradley, S. Brennan, S. Brickley, C. Brownlee, C. Courtice, N. Emptage, A. Gibb, D. Jagger, S. Keshavarzi, A. Kondratskyi, M. Lenz, S. Lilley, S. Logantha, C. Magnus, V. Marra, L. McGuinness, G. Morris, N. O'Neill, V. Piotrowski, S. Pitt, B. Potter, R. Rainbow, A. Richardson, J. Robbins, S. Schorge, P. Sharma, R. Taylor, M. Thomas, K. Torok, L. Vargova, J. Voipio, Y. Wang, J. Zorilla.
Funders, sponsors & equipment providers for 2024
CPW Liverpool 2024 would like to thank all the funders, sponsors and the generosity of companies lending equipment used on the course.
Thank you to the Company of Biologists for financially supporting the course.
Thank you to the University of Liverpool for providing the laboratory space, lecture rooms and for financially supporting the course.
Thank you to the University of Leicester for donating electrophysiology equipment to CPW.
Thank you to all the Companies for lending equipment and/or teaching during CPW: Cairn, Campden Instruments, CED, Digitimer, Nanion, Scientifica, Sophion, World Precision Instruments.
About the author
Sean Brennan is a Post-Doctoral Research Associate at the University of Liverpool, with a keen interest in the role of ion channels in health and disease. He completed his PhD in Pharmacology at the University of Manchester, where he explored the role of Kv7 ion channels in pulmonary arteries. Sean's research focuses on using phenotypic assays, patch-clamp electrophysiology, and calcium imaging to deepen our understanding of ion channels and their potential as drug targets.
- Connect with Sean on LinkedIn: https://www.linkedin.com/in/drseanbrennan/
Exclusive from Hello Bio: aCSF instant powder!
Make your electrophysiology experiments a little easier with Hello Bio's convenient aCSF instant powder packets. Ideal for sustaining ex-vivo brain sections. Simply add a packet to 1L of dH2O, mix and bubble with carbogen to make 1L of aCSF.
- Save time - no need to add Mg2+ or Ca2+
- Each pack accurately formulated - no weighing needed
- Extensively validated
Find out more here: https://hellobio.com/acsf-instant-powder-packets.html
_________________________________________________
If you enjoyed this article, why not check out the other resources available on our blog. We are passionate about supporting life scientists including early career life scientists and PhD students - with really low-priced reagents, antibodies and biochemicals, early career scientist grants, and resources to help with both personal and professional development. We know how tough it is - so we hope you find these helpful!
More General Support for Life Scientists
For advice on wellbeing, dissertations, presenting at conferences, wellbeing, PhD support, networking and lots more, we have a huge range of articles to help - just click below:
Save up to 50% on our high purity reagents...
When you get to the stage of planning your experiments, don't forget that we offer a range of low-cost, high-purity agonists, antagonists, inhibitors, activators, antibodies and fluorescent tools (yes - they really are around half the price of other suppliers!) You can use our Quick Multi-Search Tool to search for lots of products in one go, and the range includes:
- Enzyme inhibitors and activators
- Chemogenetic ligands
- Ion channel modulators
- GPCR & ionotropic receptor ligands
- Cell biology reagents & biochemicals
Technical resources
Try our Molarity Calculator: a quick and easy way to calculate the mass, volume or concentration required for making a solution.
Try our Dilution Calculator: an easy way to work out how to dilute stock solutions of known concentrations
We also offer a comprehensive range of technical resources including antibody protocols and methods, product guides and mini-reviews:
And finally, don't forget to check back in with our blog regularly for our latest articles. If there’s something you’d love to contribute to the community, whether that’s an interview or article, drop us a line at hello@hellobio.com
---