How to Choose a Secondary Antibody
This guide provides all the information you need to choose the right secondary antibody for your immunochemistry experiments. Written by our PhD qualified expert antibody team, the guide includes the factors you need to consider including purification, choosing the right conjugate, and the impact of different detection systems
Contents
1. Overview
Secondary antibodies are used to visualise the localisation of primary antibodies bound to a target through their conjugation to either a fluorophore or enzyme. There are multiple factors to consider when choosing which secondary antibody will work best for an experiment including:
- Target and host species
- Target antibody class
- Purification and/or cross-adsorption
- Conjugates
- Application used for
- Available imaging equipment
- Multiplexing
- Whether a tertiary detection system is needed
2. Choosing the right target and species
Secondary antibodies are always raised against a specific species’ antibodies. When selecting a secondary antibody you should ensure:
- The secondary antibody is raised against the same species as the primary antibody host species.
- The secondary antibody is raised against the same immunoglobulin class as the primary antibody (e.g. IgG or IgM).
3. Purification
You should ensure that the secondary antibody has been purified in a recognised way. One of the commonest purification methods is through Protein A or G affinity chromatography where Proteins A and G are bound to a resin in a chromatography column and specifically bind to immunoglobulins while other proteins from the serum are washed through.
Cross-adsorption refers to the screening of the secondary antibody against a range of non-target proteins and immunoglobulins. The secondary antibody is passed through a column containing immobilised non-target proteins with only secondaries with no cross-reactivity passing through the column. This step reduces off target binding and therefore reduces background staining in immunochemistry experiments.
4. How to choose the right conjugate
4.1 Western Blotting
The first thing to do is make yourself aware with the detection facilities available to you as this will determine which conjugate is likely to be best. The three main detection systems include:
- Enhanced chemiluminescence (ECL): Either captured using a darkroom and x-ray film or using a basic gel imaging system.
- Chromogenic detection: captured using a standard scanner.
- Fluorescence detection: captured using an advanced gel imaging system.
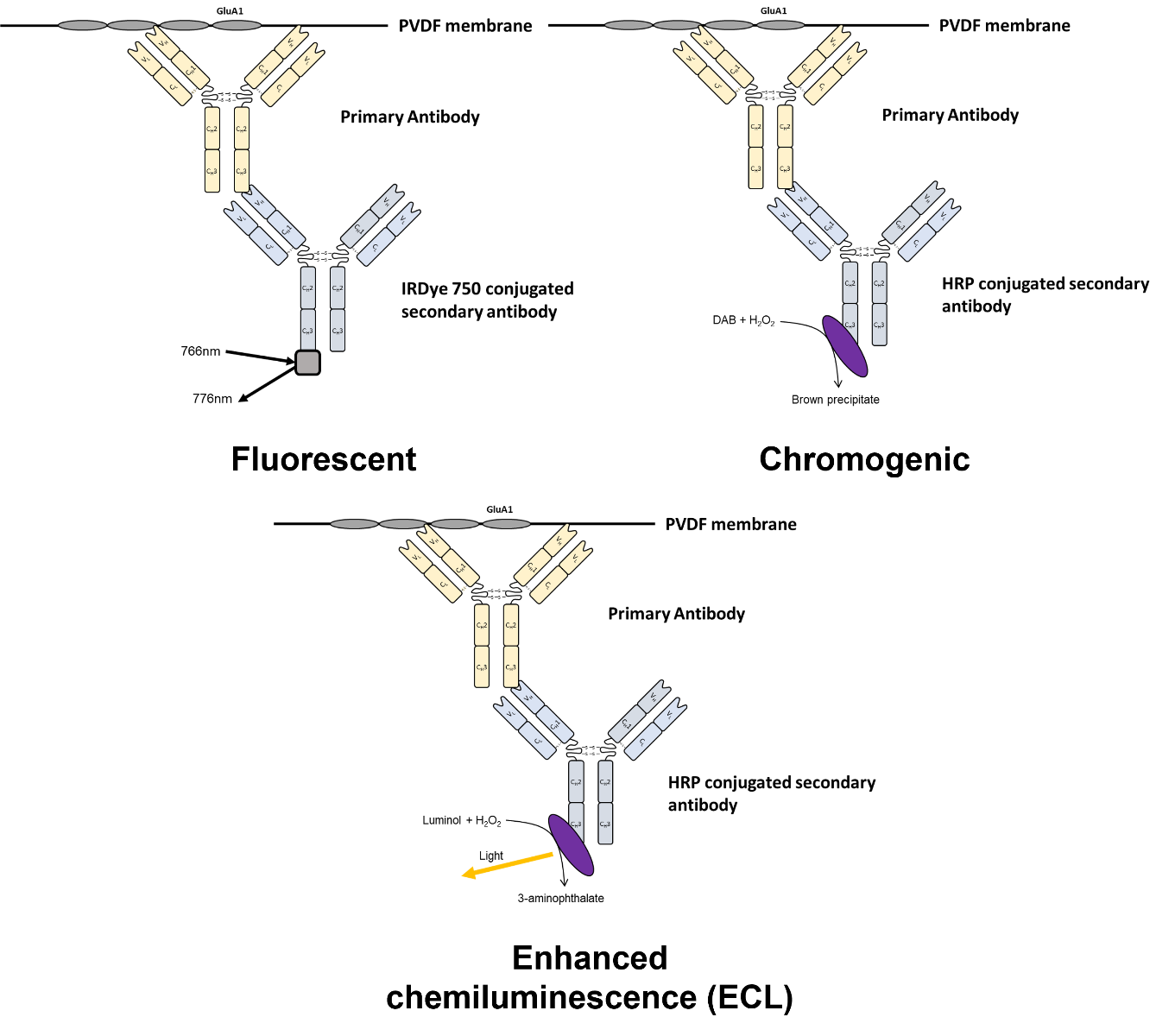
For an overview of the different detection systems see figure 1 and table 1.
Figure 1. Overview of detection methodologies available for western blotting
|
Detection method |
Secondary conjugate |
Substrates |
Considerations |
|
Enhanced chemiluminescence (ECL) |
Horseradish peroxidase (HRP) |
Luminol based ECL detection solution |
HRP based systems generally more sensitive and easier to work with |
|
Alkaline phosphatase (AP) |
Acridan and 1,2-dioxetane based ECL detection solution |
||
|
Chromogenic |
Horseradish peroxidase (HRP) |
3,3’,5,5’-tetramethylbenzidine (TMB), chloro-1-napthol (4CN) or 3, 3’-diaminobenzidine (DAB) |
Generally lower sensitivity than ECL and more difficult to work with. Allows multiplexing. |
|
Alkaline phosphatase (AP) |
nitro blue tetrazolium (NBT) or 5-bromo-4-chloro-indolyl phosphate (BCIP) |
||
|
Fluorescent |
Fluorophores (e.g. Alexa Fluor 680) |
NA |
Can multiplex and signal persists for longer than ECL |
Table 1. Summary of detection methodologies for western blotting
4.1.1 Enhanced chemiluminescence
Enhanced chemiluminescence (ECL) utilises horseradish peroxidase (HRP) or alkaline phosphatase (AP) conjugated secondary antibodies that catalyse a reaction which produces light. This is detected by either a super-cooled CCD sensor or x-ray film which enables visualisation of the blot.
HRP conjugated secondaries are most widely used due to their immediate signal generation and higher sensitivity than AP conjugated secondaries. Another consideration when using AP conjugated secondaries is that they are not compatible with phosphate buffers. HRP is inhibited by sodium azide, a commonly used preservative, therefore should not be used with solutions containing NaN3.
Due to only a single wavelength of light being emitted it is not possible to multiplex unless protein targets are present at different molecular weights whereby multiple targets can be probed for simultaneously on the same membrane (see figure 2 for example). If multiple targets are at a similar molecular weight then it is necessary to strip and re-probe the blot.

4.1.2 Chromogenic detection
Chromogenic detection utilises HRP and AP conjugated antibodies that catalyse a reaction leading to a coloured end product which precipitates onto the membrane. Common substrates for HRP are:
- 3,3’,5,5’-tetramethylbenzidine (TMB): Produces a blue product with high signal to noise
- Chloro-1-napthol (4CN): Produces a purple-blue coloured product. However issues with stability and sensitivity
- 3, 3’-diaminobenzidine (DAB): Produces a brown coloured product which can be enhanced using metal ions. Has issues however with stability.
Common substrates for AP are:
- Nitro blue tetrazolium (NBT) / 5-bromo-4-chloro-indolyl phosphate (BCIP): Produces an intense purple product which is resistant to fading.
AP reactions can be stopped by incubating with an acidic solution therefore allows multiplexing with different coloured reaction products. However AP conjugated secondaries are generally less sensitive than HRP detection systems therefore are much less frequently used. HRP is inhibited by sodium azide, a commonly used preservative, therefore should not be used with solutions containing NaN3.
4.1.3 Fluorescent detection
Fluorescent detection utilises fluorophore conjugated secondary antibodies to detect protein bands. Due to autofluorescence of PVDF at lower wavelengths, fluorophores used for WB detection tend to be highly red-shifted. A list of commonly used fluorophores in ICC/IHC and WB are detailed in table 1. Refer to imaging system specifications to find a combination of fluorophores with excitation/emission wavelengths compatible with your system.
Compared with ECL, fluorescent detection is less sensitive however allows easy multiplexing by using fluorophores with sufficiently different excitation/emission wavelengths. The fluorescence signal is also much more stable over time than an ECL signal allowing easy re-imaging while being less variable than ECL detection as it does not rely on a enzymatic reaction which may be influenced by multiple factors.
4.2 Immunohistochemistry / Immunocytochemistry
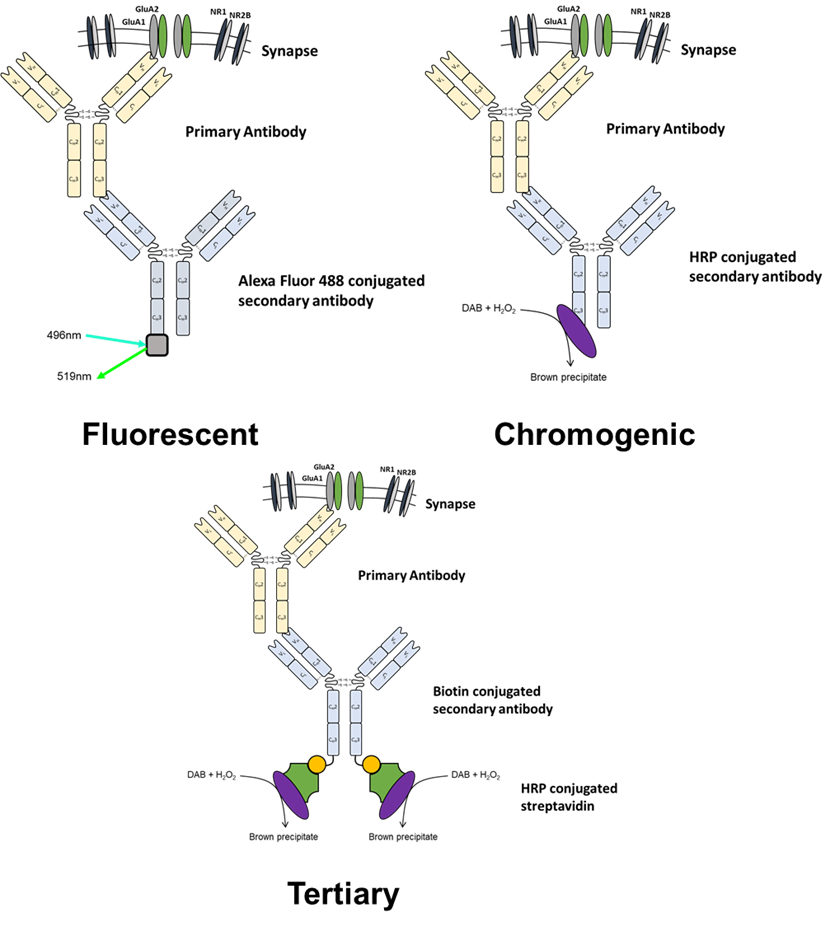
Immunohistochemistry and immunocytochemistry utilise either fluorescence or chromogenic mediated detection. The choice between methods will depend on sample preparation, experimental goals and equipment availability (see table 2 and figure 3 for an overview).
|
Detection method |
Secondary conjugates |
Advantages |
Disadvantages |
|
Chromogenic |
Horseradish peroxidase (HRP)
Alkaline Phosphatase (AP) |
Higher sensitivity due to signal amplification
Higher stability of end product allowing longer slide storage |
More time consuming
Harder to multiplex |
|
Fluorescent |
Fluorophores (e.g. Alexa Fluor 488 or DyLight 594) |
Easy multiplexing
Quicker protocol
High dynamic range |
Signal only stable for a few days
Can have issues with autofluorescence in certain tissues.
Fluorophores can photobleach |
Table 2. Overview of immunohistochemistry and immunocytochemistry detection methodologies.
Figure 3. Overview of detection methodologies available for immunohistochemistry and immunocytochemistry
4.2.1 Fluorescence mediated detection
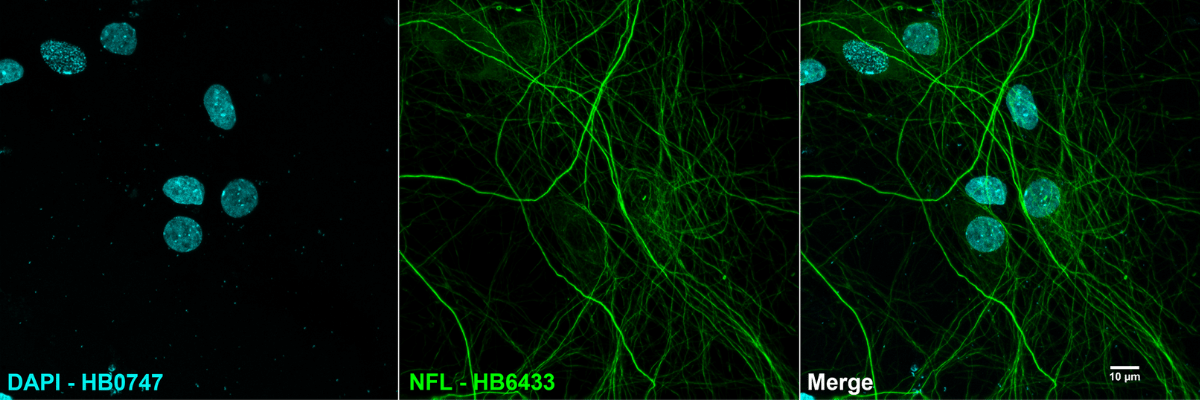
Fluorescence mediated detection is extremely popular due to its high flexibility and ease of multiplexing (see figure 4 for example). This ease of multiplexing also makes fluorescent detection suitable for analysis of co-localising proteins. The first step in choosing a fluorophore / fluorophore pairs for immunohistochemistry and immunocytochemistry is to check the specifications of the microscope that will be used for imaging. Widefield microscopes will commonly only be fitted with a limited selection of excitation and emission filters which will limit the fluorophore choices while confocal microscopes are often limited by the number of available laser channels for excitation. Excitation and emission filters are specified with a excitation and emission wavelength range which need to overlap with the excitation and emission spectra of a fluorophore. For a list of commonly used fluorophores (including counterstains) in IHC/ICC please see table 3.
Figure 4. Example of immunocytochemistry using fluorescent detection. A mouse monoclonal anti-NFL antibody (HB6433) paired with a DyLight 488 conjugated polyclonal goat anti-mouse secondary antibody was used to detect neurofilaments in cultured rat neurones utilising DAPI (HB0747) as a counterstain to visualise DNA.
|
Dye |
Maximal absorbance wavelength (nm) |
Maximal emission wavelength (nm) |
Visible Colour |
|
Alexa Fluor 350 |
346 |
442 |
Blue |
|
Hoechst 33342 |
350 |
461 |
Blue |
|
Hoechst 33258 |
352 |
461 |
Blue |
|
DyLight 350 |
353 |
432 |
Blue |
|
DAPI |
358 |
461 |
Blue |
|
DyLight 405 |
400 |
420 |
Blue |
|
Alexa Fluor 405 |
401 |
421 |
Blue |
|
Alexa Fluor 430 |
433 |
541 |
Green / Yellow |
|
EGFP |
488 |
507 |
Green |
|
Cy2 |
489 |
506 |
Green |
|
FITC |
492 |
520 |
Green |
|
DyLight 488 |
493 |
518 |
Green |
|
Alexa Fluor 488 |
496 |
519 |
Green |
|
Alexa Fluor 532 |
532 |
553 |
Yellow |
|
Cy3 |
550 |
570 |
Yellow |
|
Alexa Fluor 546 |
556 |
573 |
Orange |
|
Alexa Fluor 555 |
555 |
565 |
Orange |
|
DyLight 550 |
562 |
576 |
Orange |
|
TRITC |
557 |
576 |
Orange |
|
Alexa Fluor 568 |
578 |
603 |
Orange / Red |
|
Cy3.5 |
581 |
594 |
Orange / Red |
|
mCherry |
587 |
610 |
Red |
|
Texas Red |
589 |
615 |
Red |
|
Alexa Fluor 594 |
590 |
617 |
Red |
|
DyLight 594 |
593 |
618 |
Red |
|
Alexa Fluor 610 |
612 |
628 |
Red |
|
Alexa Fluor 633 |
632 |
647 |
Far Red |
|
DyLight 633 |
638 |
658 |
Far Red |
|
Alexa Fluor 635 |
633 |
647 |
Far Red |
|
DRAQ5 |
646 |
697 |
Far Red |
|
Alexa Fluor 647 |
650 |
665 |
Far Red |
|
Cy5 |
650 |
670 |
Far Red |
|
DyLight 650 |
652 |
672 |
Far Red |
|
Alexa Fluor 660 |
663 |
690 |
Far Red |
|
Cy5.5 |
675 |
694 |
Far Red |
|
Alexa Fluor 680 |
679 |
702 |
Near Infrared |
|
IRDye 680 |
680 |
694 |
Far Red |
|
IRDye 700 |
680 |
697 |
Far Red |
|
DyLight 680 |
692 |
712 |
Near Infrared |
|
Alexa Fluor 700 |
702 |
723 |
Near Infrared |
|
Cy7 |
743 |
767 |
Near Infrared |
|
Alexa Fluor 750 |
749 |
775 |
Near Infrared |
|
DyLight 755 |
754 |
776 |
Near Infrared |
|
IRDye 750 |
766 |
776 |
Near Infrared |
|
DyLight 800 |
777 |
794 |
Near Infrared |
|
Alexa Fluor 790 |
784 |
814 |
Near Infrared |
|
IRDye 800RS |
770 |
794 |
Near Infrared |
|
IRDye 800CW |
778 |
786 |
Near Infrared |
Table 3. Summary of some commonly used fluorophores and counterstains used in fluorescent immunohistochemistry and immunocytochemistry
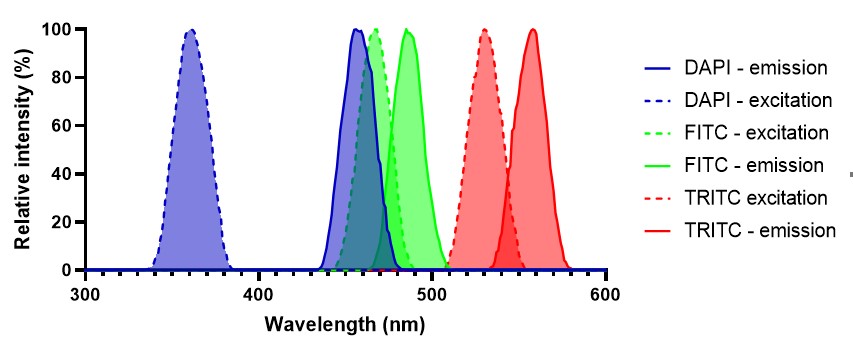
Multiplexing is achieved by using pairs of fluorophores with different excitation/emission spectra. When choosing fluorophore pairs it is critical to make sure that they don’t have overlapping excitation/emission spectra (see figure 5 for an example of a successful combination of fluorophores for multiplexing). If emission spectra of fluorophores overlap then channels can bleed into another making it difficult to tell what signal comes from what antibody. While some advanced analysis software is able to correct for this it is often helpful to have controls utilising only one fluorophore so that bleed-through can be easily detected.
Figure 5: Example of a suitable fluorophore combination for a multiplexed experiment. There is minimal overlap between excitation and emission spectra meaning that it is unlikely that signal will bleed between channels when imaging.
4.2.2 Chromogenic detection
Chromogenic detection utilises either alkaline phosphatase (AP, using 3-amino-9-ethylcarbazole (AEC) as a substrate) or horseradish peroxidase (HRP, using 3,3' diaminobenzidine (DAB) as a substrate) mediated catalysis of a reaction which results in the deposition of a coloured substrate upon the tissue section. Due to this coloured substrate not degrading it means that slides can be kept for months without an appreciable loss in signal intensity. Chromogenic detection has high sensitivity due to the enzyme reaction acting as an amplification step however is tricky to multiplex unless targets are in different cell types or locations. When this is the case then substrates with different reaction products can be used to successfully multiplex.
5. Tertiary detection
When a target is expressed at a low abundance or there are other issues with its detection then one possibility is using a tertiary detection system. These utilise the extremely high affinity between streptavidin and biotin to add another step in the immunochemistry process. Tertiary detection uses biotin conjugated secondary antibodies which are then bound to by either fluorophore or enzyme conjugated streptavidin. This adds an additional amplification step so can be useful where previous experiments using other methods of detection have been unsuccessful.