Biotin Tyramide Signal Amplification Kit Protocol
1. Product Overview
Tyramide Signal Amplification (TSA) is a technique used to amplify signals in immunofluorescence. TSA is a highly sensitive method enabling detection of low abundance proteins. TSA can easily be added into the workflow of IHC and ICC. TSA works by using a HRP conjugated secondary antibody which uses biotin tyramide as a substrate in the presence of hydrogen peroxide. Tyramide becomes activated and covalently binds to neighboring tyrosine residues, creating a dense biotin labelled region in the vicinity of the primary antibody. Fluorescently conjugated streptavidin binds with high affinity to biotin, enabling detection through immunofluorescence. TSA is also compatible with multiplexing with fluorescent secondary antibodies providing that the other antibodies used are from different species.
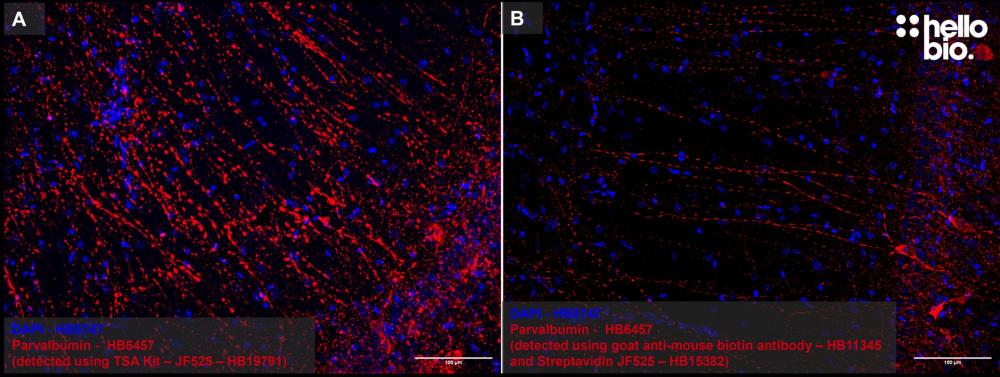
Figure 1: Representative images of Tyramide Signal Amplification compared to detection using a biotin secondary antibody with fluorescent streptavidin. A TSA of parvalbumin in the rat hippocampus using TSA kit – Janelia Fluor® 525 (HB19791). B Detection of parvalbumin using HB11345 goat anti-mouse (biotin) secondary antibody and Streptavidin Janelia Fluor® 525 (HB15382). Images were taken under identical conditions.
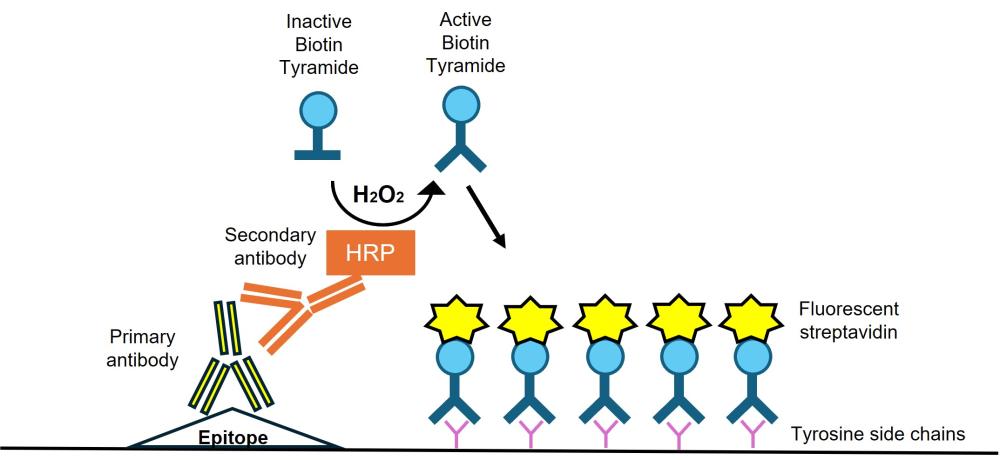
Figure 2: Diagram of Tyramide Signal Amplification. TSA uses a secondary HRP antibody which converts Biotin Tyramide from its inactive form to activated form in the presence of hydrogen peroxide. Activated Biotin Tyramide can covalently bind to tyrosine side chains on neighboring proteins. Fluorescent streptavidin can be used for detection of biotin, resulting in amplified signal.
2. Components and Storage
This kit contains:
- DMSO (100µl)
- Biotin tyramide
- Lyophilized streptavidin fluorophore conjugate
- Stop solution (100ml)
- DAPI (100µl)
Note: Please store all kit components at -20°C. Please let all components warm to room temperature before use and also note that biotin tyramide and the streptavidin fluorophore conjugate require reconstitution before use. Dissolve the biotin tyramide in DMSO (100 µl) to make a stock solution of 10 mg/ml. Aliquot and store at -20°C. Reconstitute the Streptavidin with 100 µL ddH20 to make a stock concentrationl. Store at 4°C for short term storage, or add 50% glycerol and store at -20°C for long term storage.
This kit additionally requires:
- Sodium borohydride (NaBH4) (for IHC)
- PBS (available as convenient PBS Tablets)
- Tween® 20
- Triton™ X-100 (for ICC)
- Serum (for blocking)
- Bovine serum albumin (HB5045 - available in UK only)
- Glycine (for IHC)
- H2O2
- Primary antibody (compatible with our wide range of ValidAbTM primary antibodies).
- HRP conjugated secondary antibody (check our our range of HRP conjugated secondary antibodies)
- (Optional) Avidin biotin blocking
3. Protocol
3.1 Preparing reagents and advice on optimization
Before starting experiments it is worth preparing all the necessary buffers in advance:
- PBST: 1% Tween® 20 in PBS (also available preformulated as a 20x solution - HB8088)
- Exogenous peroxidase quenching buffer: 0.3% H2O2 in PBS
- Blocking buffer: 2% BSA and 3% goat serum in PBS
- Amplification buffer: 1:10,000-1:1000 dilution biotin tyramide (this may require optimization – please see below), 0.1% Tween20, 0.003% hydrogen peroxide in PBS
It is likely that parameters will need optimizing for your specific experimental conditions. This includes:
- Concentration of primary antibody (less antibody may be required due to the amplification process, depending on the strength of the signal)
- Concentration of HRP secondary antibody
- Concentration of Biotin Tyramide (1:10,000-1:1000 dilution is recommended)
- Biotin Tyramide incubation time (2-30 minutes is recommended, cells are likely to need less time than tissue)
- Concentration of fluorescent streptavidin for detection
The concentrations of biotin tyramide and the timings given in this protocol were used for amplification of the signal from antibodies staining parvalbumin (HB6457) in free floating rat brain sections and GluA1-4 (see Nusser et al., 1998) in neuronal cell culture. It is recommended that titrations are performed to determine the optimal conditions for TSA. If excess background signal is observed this can be due to endogenous biotin in the sample. It is possible to use kits which involve incubation with avidin (to bind biotin in the tissue), followed by incubation of biotin (to bind all 4 biotin binding sites on avidin). This step should be carried out after blocking the sample. To be sure that the reaction is not occurring non-specifically, it is recommended to carry out a control in which no primary antibody is present. Any fluorescence observed in this sample would suggest an artefact of the enzymatic reaction is being observed. Further optimization of the TSA conditions as well as blocking endogenous biotin can eliminate non-specific signal.
3.2 Safety
Many of the chemicals used in immunocytochemistry and immunohistochemistry have dangerous properties and can cause serious harm if not handled correctly. Always follow local rules and read the full COSHH document for any chemical that you have not used previously. Always wear appropriate PPE such as a lab coat and gloves.
Specifically highlighted hazards:
- Formaldehyde is a highly toxic poison, skin sensitizer and carcinogen and should only ever be used in a fume hood with full PPE.
- Sodium borohydride is toxic, causes severe skin burns and eye damage in addition to reacting rigorously with H2O to create flammable gas which can ignite spontaneously.
- Hydrogen peroxide can cause irritation to the eyes, nose skin and throat.
3.3 Immunohistochemistry – Free Floating Protocol
For more information on immunohistochemistry see our immunohistochemistry protocol. 1 mL of kit components is used per well in a 12 well dish.
- Perform any antigen retrieval steps as required for primary antibodies to bind.
- Wash sections three times in PBS-Tween for 5 minutes per wash.
- Incubate slides in 1% NaBH4 in PBS-Tween for 15 minutes, RT.
- Incubate sections in exogenous peroxidase quenching buffer (0.3% H2O2 in PBS) for 15 minutes at RT.
- Wash sections three times with PBST for 5 minutes per wash.
- Incubate sections in blocking buffer (2% BSA, 3% goat serum in PBST) for 1 hour at RT.
- (Optional) To reduce background staining an endogenous biotin blocking kit can be used at this point.
- Dilute primary antibody in blocking buffer to the recommended concentration. Incubate sections at 4°C overnight on a rocker.
- Wash sections three times with PBST for 5 minutes per wash.
- Dilute the HRP secondary antibody to 4 µg/mL in blocking buffer. Incubate sections in HRP conjugated secondary for 1 hour at RT.
- Wash sections three times with PBST for 5 minutes per wash.
- Incubate sections in amplification buffer (1 mL PBST containing 0.003% H2O2, 1:1000 dilution of Biotin Tyramide, or desired concentration) for 15 minutes at RT.
- Incubate sections with 1 mL stop solution for 10 minutes at RT to quench the reaction.
- Wash sections three times with PBST for 5 minutes per wash.
- Complete steps from this point in the dark. Incubate sections with fluorescent streptavidin (1 µL of 1 mg/mL fluorescent streptavidin in 1 mL blocking buffer) for 2 hours at RT.
- Wash sections three times with PBST for 5 minutes per wash.
- Stain sections with DAPI (1:1000 DAPI in 1 mL PBS) for 10 minutes at RT.
- Wash sections with PBST for 5 minutes and then wash sections in ddH2O.
- Mount sections onto a slide using a paintbrush. Once dry, add a drop or two of mounting media and add a coverslip, taking care to avoid bubbles.
- Once the mounting media is dry store slides at 4°C in the dark until ready to image.
3.4 Immunocytochemistry Protocol
For more information on immunocytochemistry see our immunocytochemistry protocol. 1 mL of kit components is used per well in a 12 well dish.
- Permeabilize coverslips by incubating in PBS containing 0.1% Triton™ X-100 for 10 minutes at RT.
- Incubate coverslips in exogenous peroxidase quenching buffer (0.3% H2O2 in PBS) for 15 minutes at RT.
- Wash coverslips three times with PBST for 5 minutes per wash.
- Incubate coverslips in blocking buffer (2% BSA, 3% goat serum in PBST) for 1 hour at RT.
- (Optional) To reduce background staining an endogenous biotin blocking kit can be used at this point.
- Dilute primary antibody in blocking buffer to recommended concentration. Incubate coverslips at 4°C overnight.
- Wash coverslips three times with PBST for 5 minutes per wash.
- Dilute HRP conjugated secondary antibody to 1.67 ug/mL (or desired concentration) in blocking buffer for 1 hour at RT.
- Wash coverslips three times with PBST for 5 minutes per wash.
- Incubate coverslips in amplification buffer (containing 0.003% H2O2, 1:10,000 dilution Biotin Tyramide, or desired concentration, in PBST) for 2 minutes at RT.
- Incubate coverslips with stop solution for 10 minutes at RT to quench the reaction.
- Wash coverslips three times with PBST for 5 minutes per wash.
- Incubate coverslips with fluorescent streptavidin (1:1000 dilution of fluorescent streptavidin in 1 mL blocking buffer) for 1 hour at RT.
- Wash coverslips three times with PBST for 5 minutes per wash.
- Stain sections with DAPI (1:1000 dilution of DAPI in 1 mL PBS) for 2 minutes at RT.
- Wash coverslips with PBST for 5 minutes and then wash sections in ddH2O.
- Mount coverslips onto a slide using a drop of mounting media, taking care to avoid bubbles.
- Once the mounting media is dry store slides at 4°C in the dark until ready to image.
4. Troubleshooting
Tyramide signal amplification is a long multi-step process with many things that can go wrong and numerous factors that should be considered for a successful experiment. At some point it is inevitable that something will go wrong or not be optimal. Below are compiled some of the most common pitfalls that can cause sub-optimal immunohistochemistry results.
|
Problem |
Potential cause |
|
High Background
|
Too long incubation with biotin tyramide. Try reducing the incubation time and repeating the experiment. |
|
Too high concentration of HRP antibody or biotin tyramide. Try repeating the experiment with reduced concentrations of either or both. |
|
|
High levels of endogenous biotin in the sample. Try using an avidin-biotin blocking kit. |
|
|
Exogenous peroxidases have not been quenched. Ensure incubation of the sample with 0.3% H2O2 for 10 minutes prior to labeling with antibodies. |
|
|
Low signal
|
Too little primary antibody is present. Try increasing the primary antibody concentration. |
|
Too little HRP antibody is present. Try increasing the HRP antibody incubation. Ensure the HRP antibody binds to the correct species for your primary antibody. |
Please follow the above table to resolve any problems encountered when using this kit. For any problems not listed or for any further advice please contact our technical support team at technicalhelp@hellobio.com.
5. Further Reading
Tyramide Signal Amplification for Immunofluorescent Enhancement. Faget L et al (2015) Methods in molecular biology (Clifton, N.J.) 1318 : 161-72. Read more (PubMedID: 26160574)
Application of tyramide signal amplification system to immunohistochemistry: a potent method to localize antigens that are not detectable by ordinary method. Toda Y et al (1999) Pathology international 49 : 479-83 Read more (PubMedID: 10417696)
Amplification of fluorescent in situ hybridisation signals in formalin fixed paraffin wax embedded sections of colon tumour using biotinylated tyramide. McKay JA et al (1997) Molecular pathology : MP 50 : 322-5 Read more (PubMedID: 9536283)